Tender Timeline
Tender Title: Diagnostic Consumables
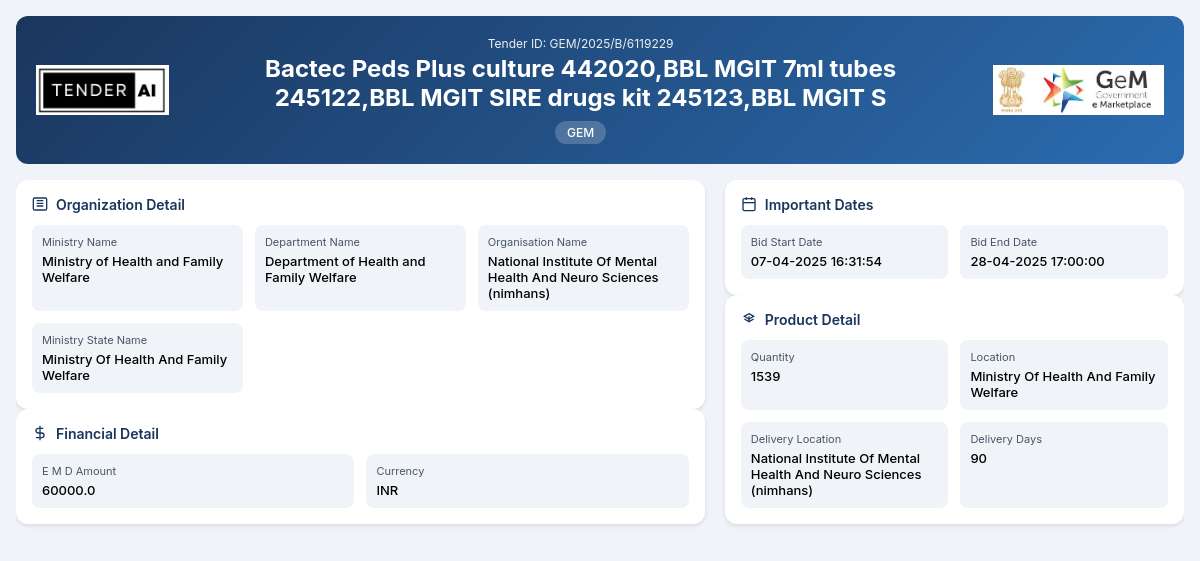
Tender Reference Number: GEM/2025/B/6119229
Issuing Authority/Department: Department of Health and Family Welfare, Ministry of Health and Family Welfare
Overview
The Diagnostic Consumables tender aims to procure essential medical supplies critical for healthcare facilities across the nation. This tender invites qualified suppliers to submit bids for various diagnostic consumables, emphasizing the importance of quality and compliance with health regulations. The total quantity required for this procurement is 1,539 units, encompassing a range of products essential for accurate diagnosis and treatment.
Scope of Work and Objectives
The scope of work includes the supply of various diagnostic consumables classified under the categories such as Bactec Peds Plus culture (442020), BBL MGIT 7ml tubes (245122), BBL MGIT SIRE drugs kit (245123), and additional products required for efficient and reliable diagnostic processes. The objective of the tender is to ensure healthcare facilities have access to high-quality diagnostic tools while adhering to the safety and health standards as stipulated by governmental bodies.
Eligibility Criteria
To participate in this tender, bidders must meet specific eligibility requirements which include being a registered entity competent to supply the required items. Suppliers should also demonstrate a history of past performance in supplying similar products to healthcare institutions.
Technical Requirements
Bidders must comply with stringent technical requirements that align with industry standards for diagnostic equipment. This includes certifications regarding product efficacy and safety, as well as adherence to quality assurance protocols. All products must be manufactured in facilities that comply with relevant regulations.
Financial Requirements
Each bidder is required to provide financial documentation demonstrating their stability and capability to fulfill the tender requirements. This includes providing proof of transactions, balance sheets, and any licensing documents relevant to the business operations in the health sector.
Document Submission Details
Interested suppliers must submit their bids in accordance with the specified guidelines for electronic submission. All documents should be in acceptable formats as outlined in the tender requirements and must be submitted before the deadline stipulated in the tender announcement.
Special Provisions
The tender includes special provisions aimed at encouraging participation from Micro, Small, and Medium Enterprises (MSEs) and startups. Such provisions may include relaxation of financial criteria or technical requirements to promote inclusivity within the healthcare supply chain.
Evaluation Process
The evaluation process will consider several parameters including technical specifications, financial bids, compliance requirements, and overall responsiveness to the tender documents. A well-defined evaluation matrix will be utilized to ensure a fair and transparent selection process.
Delivery Locations
Products must be delivered to designated healthcare facilities as specified by the Department of Health and Family Welfare. Bidders should ensure that their delivery frameworks can accommodate the logistical needs inherent in healthcare supply and provision.
Contact Information
For further inquiries, bidders may reach out to the Department of Health and Family Welfare via the official email associated with the tender reference. Detailed contact information is available in the tender documents for direct communication.
In conclusion, the GEM/2025/B/6119229 tender presents a vital opportunity for suppliers of diagnostic consumables to engage with the public health sector. By contributing high-quality products, bidders play a crucial role in enhancing health services across the nation, ensuring that medical institutions are equipped with the necessary tools to deliver exemplary patient care.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
5 DocumentsFrequently Asked Questions
The eligibility requirements include being a registered entity capable of supplying medical consumables. Bidders must demonstrate prior experience or capability in providing similar products to healthcare institutions. Compliance with all applicable health regulations is also essential.
In this tender, products are required to align with stringent technical specifications emphasizing quality assurance. This includes certifications for safety, efficacy, and compliance with health industry standards, ensuring that all diagnostic consumables are reliable and pose no risk to users.
Bidders must submit their proposals electronically, adhering to the guidelines set forth in the tender documents. Accepted document formats typically include PDFs, Word documents, and Excel spreadsheets. It’s crucial for bidders to confirm that they utilize the specified formats to avoid disqualification.
The Earnest Money Deposit (EMD) is a security measure that confirms a bidder’s commitment to the tender process. Details about the EMD amount, payment methods, and conditions for refund can be found in the tender documentation. It is essential for bidders to comply with these terms to participate.
Yes, the tender provides special provisions designed to support Micro, Small, and Medium Enterprises (MSEs) and startups. These benefits may include preferential evaluation criteria, support in meeting technical and financial thresholds, as well as potential exemptions to encourage participation from smaller suppliers.
In conclusion, the GEM/2025/B/6119229 tender offers an opportunity for suppliers to engage with the healthcare sector while promoting inclusivity and quality in medical supplies.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders