Department of Science and Technology (DST) Tender by Sree Chitra Tirunal Institute For Medical Sciences And Technology (sctimst) (GEM/2025/B/6109668)
DSHVALV0169,DSHVLV0170,DSHVLV0173,DSHVLV168,DSHVLV0171,DSHVLV0172
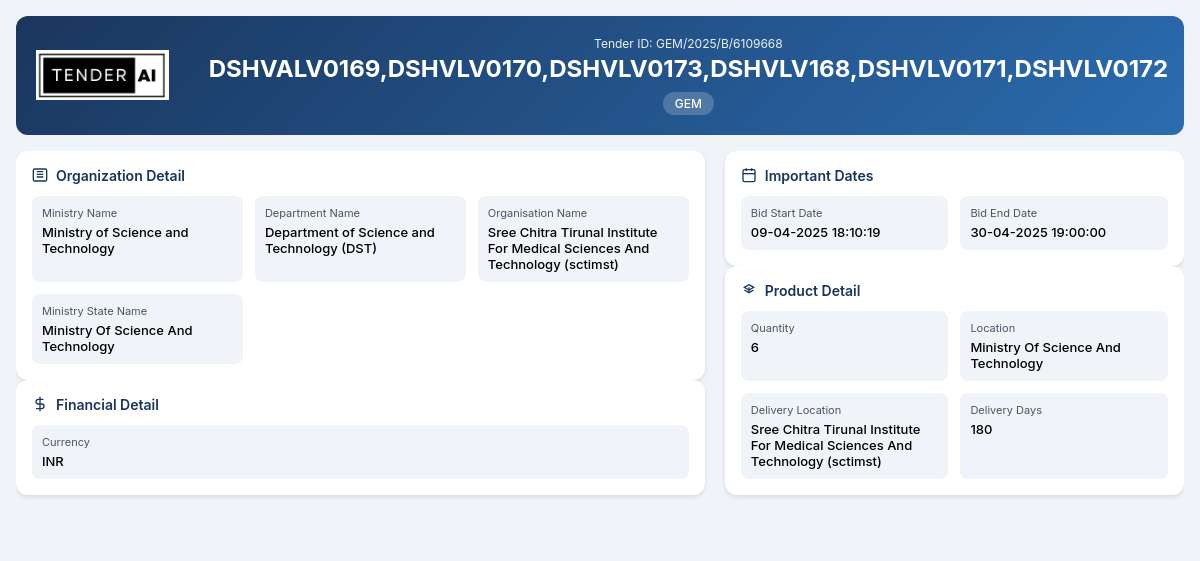
Tender Timeline
Tender Title
INSPIRIS RESILIA HEART VALVE
Reference Number: GEM/2025/B/6109668
Issuing Authority
Department of Science and Technology (DST)
Ministry of Science and Technology
Scope of Work and Objectives
The goal of this tender is to procure the INSPIRIS RESILIA HEART VALVE, a critical device in the field of cardiac surgery. The heart valve is intended for use in medical institutions for patients requiring valve replacement due to heart ailments. The tender aims to ensure that only high-quality valves are supplied, enhancing patient safety and outcomes. By securing this tender, the Department of Science and Technology aims to align with healthcare standards and improve technological advancement within the medical sector.
Eligibility Criteria
Bidders must meet specific eligibility requirements to participate in this tender. Eligible entities include registered manufacturers or authorized distributors of heart valves. To qualify, bidders should have a proven track record in medical device supply and quality assurance, alongside necessary certifications and compliance with local and international health regulations.
Technical Requirements
Participants must provide technical specifications demonstrating adherence to stringent quality standards set by regulatory authorities for heart valves. All products must showcase compliance with recognized testing criteria related to durability, functionality, and safety. Bidders are required to submit detailed documentation regarding manufacturing processes and quality control measures in place.
Financial Requirements
The bidding entities must confirm their financial capability to deliver the required heart valves. This involves providing proof of previous contracts, including values, and other relevant financial documentation that projects sustainability and reliability in fulfilling the contract terms.
Document Submission Details
Bidders are required to submit all necessary documents electronically via the designated online portal. This includes bids, certificates of registration, proof of eligibility, financial statements, and technical documentation. All submissions must adhere to the accepted formats as stated in the tender guidelines.
Special Provisions
This tender includes special provisions for Micro, Small, and Medium Enterprises (MSEs), encouraging their participation in national health advancements. Furthermore, startups fulfilling the eligibility standards will receive favorable consideration to boost innovation within the healthcare sector.
Evaluation Process
Bids will undergo a thorough evaluation process based on criteria such as technical compliance, financial stability, and the overall cost-effectiveness of the proposal. The scoring will involve a combination of qualitative and quantitative assessments to identify the most potential bidders who can meet the health industry demands effectively.
Delivery Locations
The delivery locations for the heart valves will primarily be designated hospitals and clinics across various states as specified by the ministry's guidelines. Bidders should ensure their logistics plan accommodates these locations with reliability and time efficiency.
Contact Information
For further inquiries regarding the tender details, potential participants may contact the Department of Science and Technology through the designated contact channels provided in the official documentation.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
5 DocumentsDocuments Required from Seller
- Certificate (Requested in ATC)
- OEM Authorization Certificate *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Frequently Asked Questions
The eligibility requirements for participating in this tender include being a registered entity recognized for the manufacture and distribution of medical devices. Participants must demonstrate compliance with healthcare regulations, have previous experience in supplying cardiac devices, and provide necessary certifications to prove technical and financial competency.
Bidders must submit various certificates including quality management system endorsements (such as ISO certification) and product-specific certifications that prove the heart valves meet the health standards mandated by regulatory authorities. Validity of these documents is crucial for ensuring compliance and enhancing the credibility of the bid.
The registration process can vary depending on the entity’s preparedness and the completeness of their submitted documentation. Typically, interested organizations should plan for a timeframe that allows for gathering required documents, registering on the tender portal, and resolving any potential queries to ensure a smooth submission before the deadline.
Bidders must ensure that submitted documents are in accepted document formats as specified in the tender guidelines. Generally, this includes PDF files for documents, spreadsheets for quoting items, and any additional formats that support necessary information efficiently. Compliance with format requirements is critical for successful bid consideration.
The evaluation and selection process involves a comprehensive review of all submitted bids according to predefined criteria including technical specifications, financial capability, and compliance with health regulations. Selected bidders will be notified, and results will be available through designated communication channels as outlined in the tender announcement.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders