Supply of Video Laryngoscope with Endoscope and Chest Holder
Video laryngscope with Endoscope and chest holder
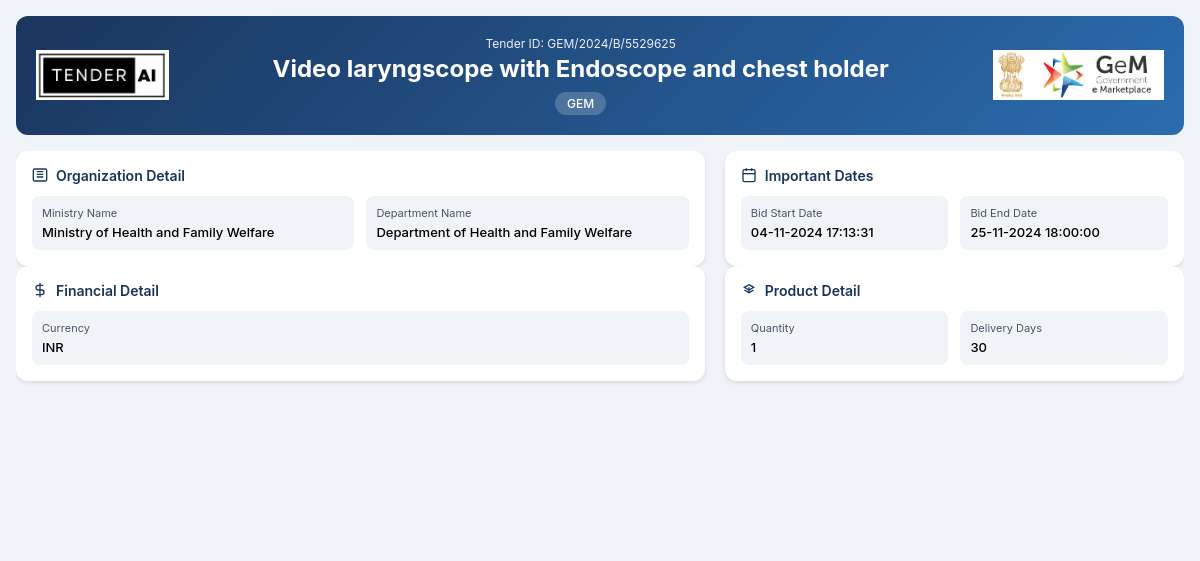
Tender Timeline
Tender Title: Supply of Video Laryngoscope with Endoscope and Chest Holder
Reference Number: 2f5ae7836
Issuing Authority/Department
Department of Health and Family Welfare
Ministry of Health and Family Welfare
Scope of Work and Objectives
The objective of this tender is to procure a Video Laryngoscope with Endoscope and Chest Holder to enhance medical service delivery in healthcare settings. The successful bidder will be required to supply the product that meets the specifications outlined by the Department of Health and Family Welfare. The device must be suitable for the intended medical applications, ensuring safety and efficacy in patient care.
Eligibility Criteria
Bidders must meet the following eligibility requirements:
- Must be a registered entity authorized to supply medical equipment in India.
- Must possess a minimum average annual turnover of 4 Lakh (INR) for the last three financial years.
- Compliance with specified documentation, including OEM Authorization Certificate and supporting documents attesting to experience criteria.
- Micro, Small, and Medium Enterprises (MSEs) and start-ups are eligible for exemptions regarding years of experience.
Technical Requirements
The technical specifications for the video laryngoscope include:
- High-resolution imaging capability.
- Endoscope compatibility.
- Chest holder stability and safety features.
- Conformity to relevant medical equipment regulations and quality standards.
Financial Requirements
Bidders are required to submit an Earnest Money Deposit (EMD) of 14,000 INR along with their bid. The bid must also comply with the financial guidelines laid out in the tender document. Additionally, the financial evaluation will be based on the total value-wise evaluation.
Document Submission Details
Bids must be submitted electronically via the GeM portal. Required documents include:
- Experience Criteria
- Bidder Turnover
- OEM Authorization Certificate
- Compliance of BoQ specifications
In case of seeking exemptions from experience or turnover criteria, the relevant supporting documents must be uploaded for assessment by the buyer.
Special Provisions
This tender provides special considerations for MSEs and Startups, allowing for exemptions from the standard experience and turnover requirements. This initiative aims to promote inclusivity within the tendering process and enhances opportunities for smaller entities.
Evaluation Process
The evaluation for bids will be based on both technical compliance and financial bids. Bidders must meet the technical specifications and will be evaluated based on their qualification under the following criteria:
- Total value-wise evaluation methodology to ensure best value for money.
- Notification of results will be sent to all bidders post-evaluation.
Delivery Locations
Successful bidders will need to ensure timely delivery to specified healthcare facilities as directed by the Department of Health and Family Welfare. Details regarding exact delivery locations will be provided upon contract finalization.
Contact Information
For further inquiries, interested bidders may refer to the official documents linked below for any clarifications required regarding the tender specifications.
This tender presents a noteworthy opportunity for suppliers to contribute to the healthcare sector by delivering essential medical equipment that aligns with current healthcare standards and regulations.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Corrigendum Updates
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity authorized to supply medical equipment in India, with a minimum average annual turnover of 4 Lakh (INR) for the last three years. MSEs and startups are eligible for exemptions regarding years of experience.
Bidders must submit various documents, including the OEM Authorization Certificate, evidence of experience criteria, and Bidder Turnover documents. Compliance with the BoQ specification is also mandatory.
To participate, bidders must register on the GeM portal and submit the required documents electronically as specified in the tender guidelines.
Documents should be submitted in PDF or any specified format as detailed in the tender document. Ensure all files are clear and legible to facilitate evaluation.
The technical specifications include features such as high-resolution imaging, compatibility with endoscopes, and safety measures integrated into the chest holder. All products must comply with medical equipment standards.
Yes, all submissions must comply with established national and international quality standards for medical devices to ensure the safety and efficacy of the product.
Bidders need to comply with relevant procurement policies, including guidelines set forth by the Ministry of Health and Family Welfare, as well as any quality certifications required for medical equipment.
The supplied products will undergo rigorous evaluations to ensure they meet specified technical and quality standards as detailed in the tender documents.
A 14,000 INR EMD must be submitted along with the bid documents. This deposit secures the bid and is returned to unsuccessful bidders post-evaluation.
Performance security will be required from the successful bidder to ensure compliance with the contract terms upon awarding the bid.
Payment terms will be outlined in the tender document, generally involving a procedure for invoicing upon delivery and acceptance of the products.
The price evaluation will be based on the total value of the bid, ensuring that the best value for money is obtained while considering quality and compliance factors.
Bids must be submitted electronically on the GeM portal, adhering to the submission guidelines provided in the tender documents to ensure compliance.
While specific dates are not included, all bidders should refer to the official tender document for the timeline regarding submission deadlines and other important dates.
The evaluation process involves assessing both technical compliance and financial bids, ensuring that technically qualified bidders are considered for the awarding process.
Notification of results will be communicated to all bidders who have submitted their proposals, detailing the outcome of the evaluation and any further steps required in the process.
MSEs are eligible for exemptions from certain eligibility criteria, promoting inclusivity and ensuring they have the opportunity to participate in significant contracts.
Startups also benefit from exemptions similar to those offered to MSEs, encouraging innovation and participation in procurement processes by new entrants to the market.
Participants are encouraged to align with the ‘Make in India’ policies, promoting local manufacturing and content in products supplied through this tender process.
Bidders must comply with local content rules as specified in procurement guidelines, aiming to support domestic manufacturing and economic development.
By addressing these frequently asked questions, potential bidders can gain better insight into the tender process, requirements, and benefits, ensuring a well-informed submission strategy.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders