Department of Military Affairs Tender by Indian Army (152a067d8)
Coronary Imaging catheter for Intra Vascular Ultrasound 40 MHz compatible,Coronary Orbital Atherect
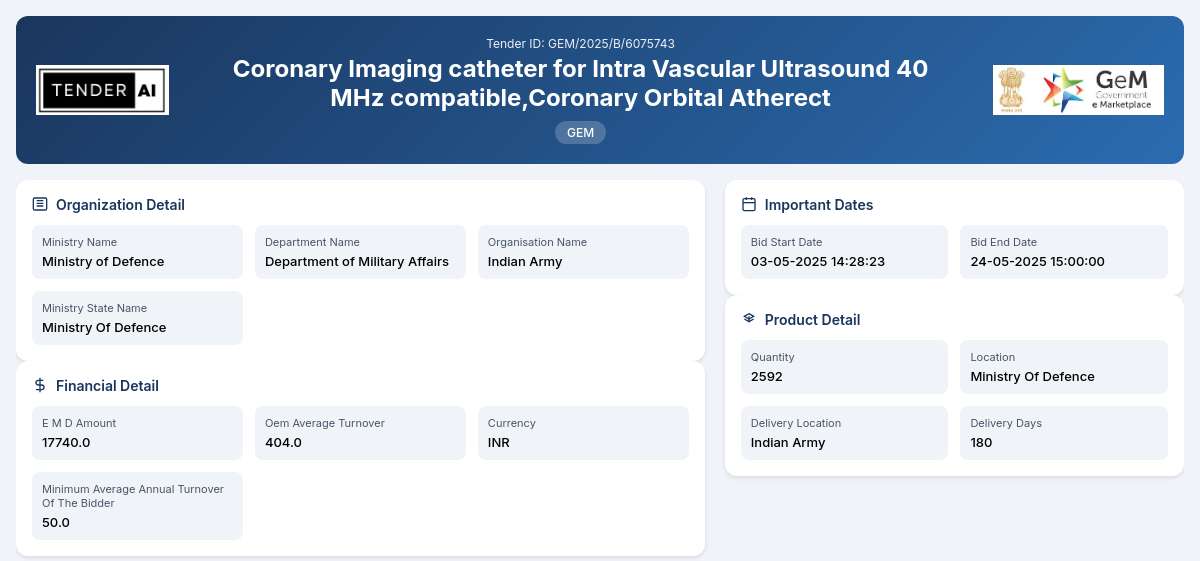
Tender Timeline
Tender Title: IFA 60
Reference Number: 152a067d8
Issuing Authority/Department:
Ministry of Defence, Department of Military Affairs
Scope of Work and Objectives:
The objective of this tender is to procure Coronary Imaging Catheters compatible with Intra Vascular Ultrasound operating at 40 MHz, as well as Coronary Orbital Atherectomy Systems with associated glide assist accessories. The total quantity required for this tender is 2592 units. This procurement aims to enhance the medical equipment capabilities within military medical facilities, ensuring the availability of advanced cardiac interventions for personnel.
Eligibility Criteria:
Interested bidders must be registered entities capable of delivering the specified medical devices. Companies must demonstrate experience in manufacturing or supplying medical equipment similar to the Coronary Imaging Catheters and Atherectomy Systems and must comply with local and international regulations pertaining to healthcare products.
Technical Requirements:
Bidders are required to provide detailed specifications of the Coronary Imaging Catheters, focusing on compatibility, safety standards, and operational efficacy. Additionally, all offered products must comply with recognized healthcare standards and be proven effective through prior testing. Certificates and documentation evidencing compliance with technical specifications must accompany the bid.
Financial Requirements:
Bidders must submit a comprehensive financial proposal detailing the cost per unit and total pricing for the 2592 units. The proposal should comply with all specified payment terms, which will be discussed during negotiations upon the tender's initial evaluation.
Document Submission Details:
Submissions must be prepared in the requested format and submitted to the designated tender submission platform established by the Ministry of Defence. Bidders are encouraged to ensure all documents are comprehensive and clearly organized. Any incomplete submissions may lead to disqualification.
Special Provisions:
This tender encourages participation from Micro, Small, and Medium Enterprises (MSEs) and Startups. Bidders that qualify as MSEs may benefit from relaxed eligibility criteria and other supportive measures aimed at promoting small-scale enterprise procurement.
Evaluation Process:
The evaluation process will encompass both technical and financial assessments. Technical proposals will be reviewed for compliance with quality standards, while financial proposals will be scrutinized for competitiveness. Considerations for local content and adherence to 'Make in India' policies will also play a crucial role in the evaluation.
Delivery Locations:
Successful bidders will be required to deliver the products to designated military medical facilities, whose specifics will be outlined at the time of contract award.
Contact Information:
For questions and clarifications, bidders can refer to the official contact details provided in the tender documentation.
Tender BOQ Items
12 ItemsGeneral Information
Financial Information
Evaluation and Technical Information
Tender Documents
5 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity with the capability to supply the specified medical devices. Bidders should have prior experience in manufacturing or supplying products similar to the Coronary Imaging Catheters and Coronary Orbital Atherectomy Systems. Compliance with local laws and healthcare regulations is mandatory.
Bidders must provide certificates that demonstrate compliance with technical specifications and industry quality standards. Documentation validating the effectiveness and safety of the Coronary Imaging Catheters and Atherectomy Systems should also be included in the submission to adhere to compliance requirements.
To participate, bidders must register as a legitimate entity on the designated procurement platform indicated by the Ministry of Defence. After registration, bidders can access the detailed tender documentation and prepare their submission in accordance with the outlined submission methods and document formats.
Documents must be submitted in formats specified within the tender guidelines. Commonly accepted formats include PDF for formal proposals and any spreadsheet formats for financial breakdowns. It is crucial that all submissions are clearly legible and professionally formatted to facilitate effective evaluation.
MSEs stand to benefit from specially tailored provisions within this tender aimed at encouraging their participation. These may include relaxed eligibility requirements, guidance through the bidding process, and additional support to foster engagement in government procurements. Compliance with Make in India policies, emphasizing local manufacturing and sourcing, is highly valued.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders