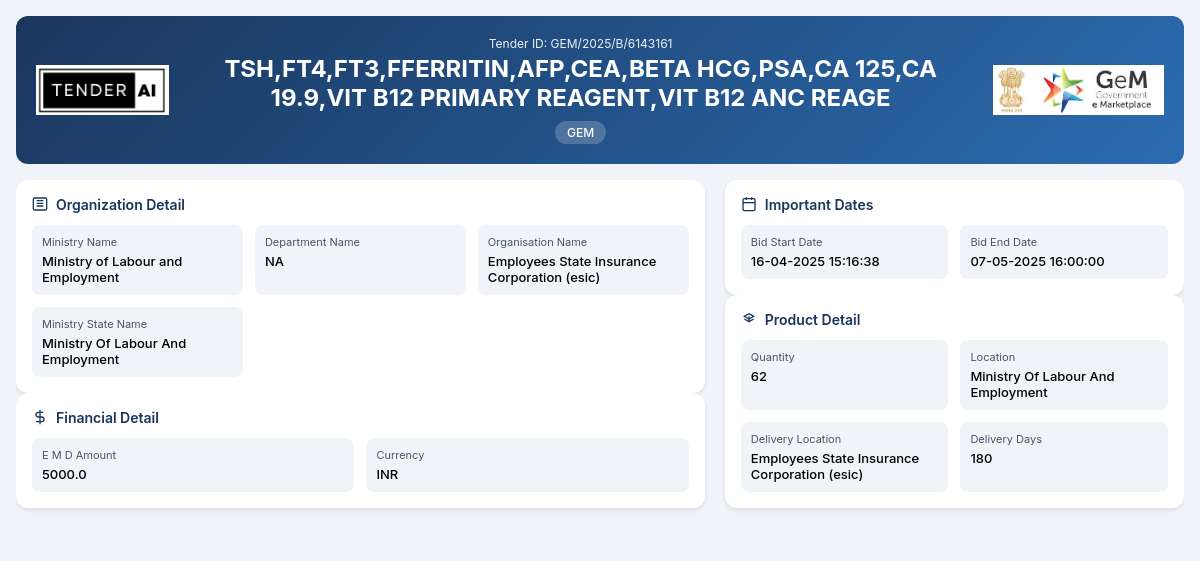
Tender Title: Supply of Laboratory Reagents
Tender Reference Number: GEM/2025/B/6143161
Issuing Authority: Ministry of Labour and Employment
Scope of Work and Objectives
The primary objective of this tender is to procure a variety of laboratory reagents essential for conducting various medical tests. This includes but is not limited to, reagents required for tests related to thyroid function (TSH, FT4, FT3), tumor markers (CEA, PSA, CA 125, CA 19.9), as well as vitamin assessments (VIT B12 PRIMARY REAGENT, VIT B12 ANC REAGENT, VIT B12 DTT RELEASING AGENT). The expected total quantity for this tender is 62 units.
The tender seeks suppliers who can fulfill the requirements with adherence to quality standards while aiming for timely delivery to facilitate uninterrupted lab operations.
Eligibility Criteria
To be eligible to participate in this tender, the bidder must be a registered entity capable of supplying the specified reagents. Bidders should have prior experience in providing similar products and holding relevant certifications that confirm their ability to meet required specifications.
Technical Requirements
The tender require the suppliers to meet quality specifications laid out for each reagent type. All proposed products should comply with the prescribed testing criteria and quality standards relevant to laboratory practices. Necessary documentation to prove compliance with these technical specifications will be required upon submission of the bid.
Financial Requirements
Bidders must demonstrate their financial capability to undertake the contract. This may include providing evidence of previous contracts successfully completed and any relevant financial statements. An Earnest Money Deposit (EMD) may be required to ensure seriousness in bids, the specifics of which will be revealed in the bid documentation.
Document Submission Details
All bids must be submitted electronically through the designated procurement portal. Detailed instructions outlining the required formats for submission—such as PDF or Excel—will be provided to ensure clarity in the submission process. Bidders are advised to carefully follow the guidelines to avoid disqualification.
Special Provisions
Special considerations may be provided for Micro, Small, and Medium Enterprises (MSEs) and startups, as defined by government policy. Favorable terms and conditions may be applied for these entities to encourage participation in the tender process.
Evaluation Process
The evaluation of submitted bids will be conducted based on predefined criteria including compliance with technical specifications, pricing evaluations, and financial stability. Each bidder's proposal will be scored and ranked accordingly, and the highest-ranked bidder will be awarded the contract.
Delivery Locations
Upon award of the tender, the successful bidder will be required to deliver the reagents to specific laboratories and testing facilities as indicated in the bid documents. Timeliness and thoroughness will be essential in fulfilling delivery requirements.
Contact Information
For more insights or clarification, bidders can reach out to the designated department within the Ministry of Labour and Employment. Official contact details may be available upon further review of the tender documents.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
5 DocumentsDocuments Required from Seller
- Experience Criteria
- OEM Authorization Certificate
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity capable of providing the specified laboratory reagents. Bidders should have relevant certifications and prior experience in the supply of similar products to ensure compliance with quality standards and to demonstrate capability in fulfilling the tender requirements.
The technical specifications require compliance with standard laboratory practices, meaning that all reagents must meet quality assurance criteria and relevant health regulations. Bidders are expected to provide documentation that verifies adherence to these standards, along with details of testing criteria if applicable.
Yes, the registration process involves submitting required documentation and certifications to the designated procurement portal. Bidders must ensure they complete all registration steps correctly, including the electronic submission of necessary documents in accepted formats to qualify for participation in the tender.
The Earnest Money Deposit (EMD) is a security deposit that bidders submit along with their bids to demonstrate commitment to the proposal. The specific amount to be deposited will be detailed in the tender documents, which will be essential for participating in the bidding process.
The evaluation process involves assessing submitted proposals based on a scoring system that considers technical compliance, financial viability, and pricing. Each submission will be thoroughly reviewed, and selectors will ensure that necessary quality standards and other criteria outlined in the tender documents are met before determining the winning bid.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders