Department of Health Research Tender by Indian Council Of Medical Research (icmr) (GEM/2025/B/6140047)
Viral Transport Medium (VTM) Kit
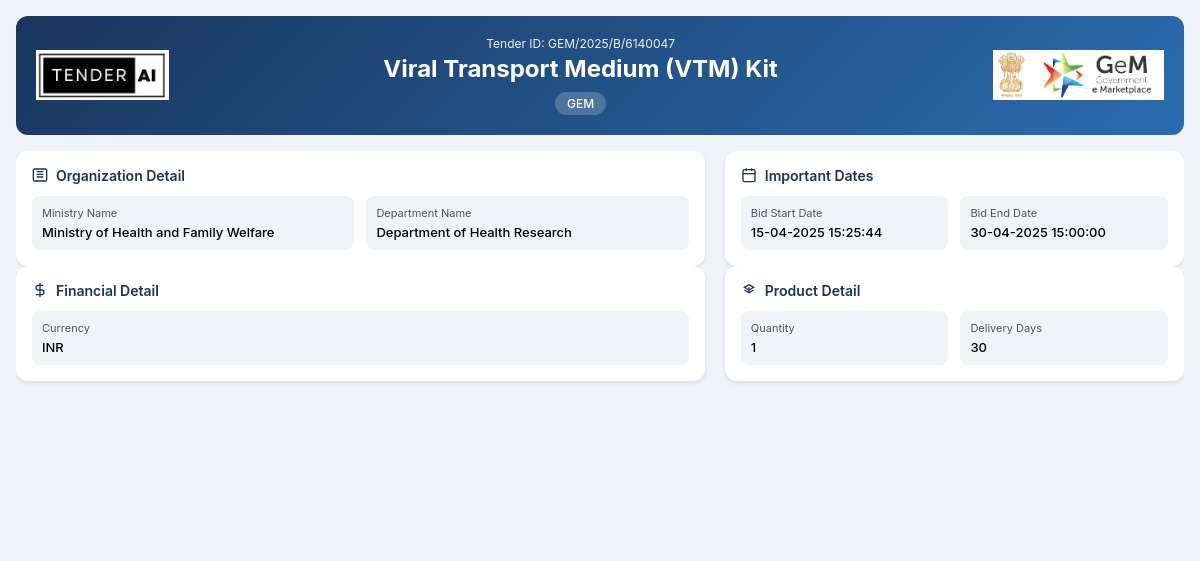
Tender Timeline
The Tender for Viral Transport Medium (VTM) Kit, referenced as GEM/2025/B/6140047, is issued by the Department of Health Research, under the Ministry of Health and Family Welfare. This tender aims to procure a total of 40,000 units of Viral Transport Medium (VTM) Kits to support the ongoing health initiatives and enhance the capacity for managing viral testing.
Scope of Work and Objectives
The primary objective of this tender is to secure high-quality VTM Kits that meet specific technical specifications necessary for preserving viral specimens during storage and transport. The procured kits will play a crucial role in the testing and diagnosis of various viral infections, thus forming an integral part of the public health response.
Eligibility Criteria
To participate in this tender, bidders must possess the necessary qualifications, including being a registered entity in the relevant domain. It is essential for bidders to demonstrate a history of supplying medical and laboratory equipment, maintaining compliance with health regulations.
Technical Requirements
The Viral Transport Medium (VTM) Kit must adhere to strict technical specifications. Bidders are required to submit these technical specifications along with any relevant documentation, including quality certificates confirming compliance with national and international standards.
Financial Requirements
Bidders are required to provide a complete financial proposal outlining the cost per unit along with any additional charges. This proposal must also include compliance with payment terms as specified in the tender document.
Document Submission Details
All documents related to this tender must be submitted electronically via the designated portal. Bidders are required to ensure that all required documents are complete and comply with submission guidelines to avoid disqualification.
Special Provisions
The tender encourages participation from Micro, Small, and Medium Enterprises (MSEs) as well as startups. Special provisions may be offered to enhance their participation and competition in the tender process.
Evaluation Process
Bids will be evaluated based on a multi-criteria approach, considering both technical and financial aspects. The evaluation will ensure fairness and transparency in awarding the contract, with a focus on the ability to meet delivery timelines and product quality.
Delivery Locations
The successful bidder will be required to deliver the VTM Kits to specified locations across the country as delineated during the award process. Timely delivery is critical for the effective functioning of testing facilities.
Contact Information
For further questions regarding this tender, parties are encouraged to reach out through the appropriate channels listed in the official tender portal. It is recommended to clarify any doubts early in the submission process.
In summary, this tender represents a significant opportunity for eligible suppliers to contribute to critical health initiatives by providing essential Viral Transport Medium Kits. The emphasis will be placed on compliance with quality, timely delivery, and the ability to meet the immediate demands of health authorities.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- OEM Authorization Certificate *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity with relevant experience in the medical supply sector. Participants must possess the necessary paperwork that demonstrates compliance with the tender specifications and regulatory standards.
The technical specifications for the VTM Kits necessitate adherence to industry standards for viral specimen preservation. This includes the type of mediums used, sterility, and compliance with relevant health guidelines. Interested bidders should provide detailed documentation regarding these specifications.
The Earnest Money Deposit (EMD) required for this tender is a critical component for maintaining the seriousness of bidders. The specific amount will be detailed in the tender document, and it must be submitted along with the financial proposal to ensure consideration.
All submission methods must comply with electronic formats specified in the tender documentation. Bidders must ensure that all documents are submitted via the official portal to meet the requirements and deadlines.
Yes, there are specific provisions for MSEs and startups which aim to facilitate greater participation in the tendering process. These provisions can include relaxed qualification criteria and potential financial benefits, making it easier for smaller entities to compete.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders