Department of Health and Family Welfare Tender by Jawaharlal Institute Of Postgraduate Medical Education And Research (jipmer) (GEM/2025/B/6164109)
ELECTRICALLY OPERATED CARDIAC COMPRESSION DEVICE
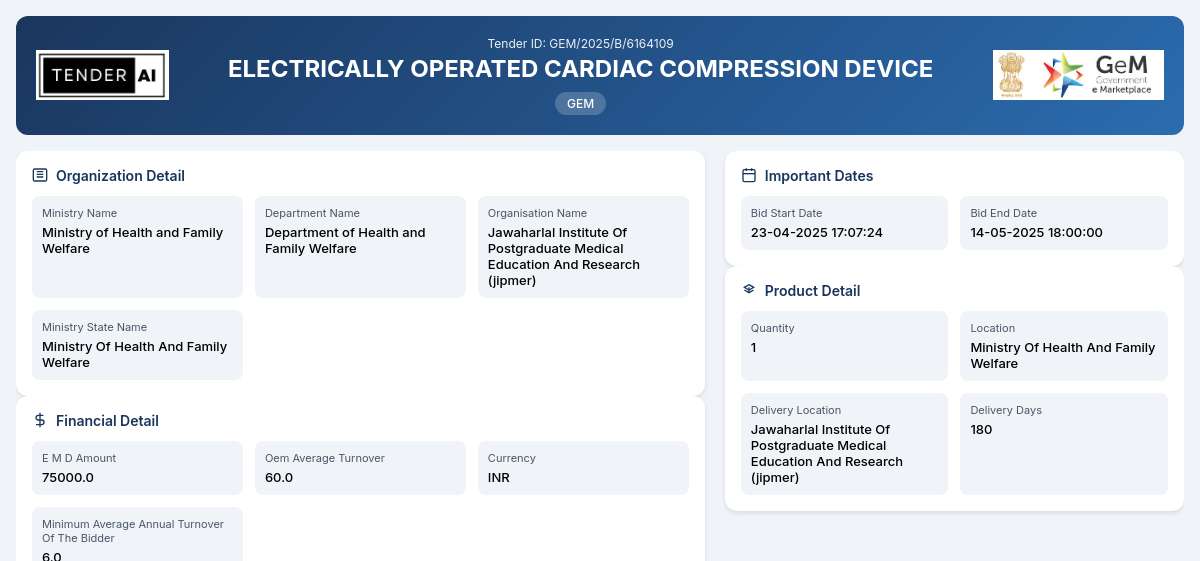
Tender Timeline
Tender Title: Electrically Operated Cardiac Compression Device
Tender Reference Number: GEM/2025/B/6164109
Issuing Authority/Department: Department of Health and Family Welfare, Ministry of Health and Family Welfare
Scope of Work and Objectives
The primary objective of this tender is to procure Electrically Operated Cardiac Compression Devices. These specialized medical devices are vital for enhancing cardiac resuscitation techniques in critical care environments, particularly in hospitals and emergency medical services. The successful bidder is expected to supply high-quality compression devices that adhere to the necessary technical specifications, ensuring maximum safety and efficacy for patient care.
Eligibility Criteria
Potential bidders must meet specific eligibility requirements to participate in this tender process. The eligibility criteria include:
- Registration as a legal entity in the relevant medical device sector.
- Valid certifications indicating compliance with national and international health and safety standards.
- Proven experience in the supply of medical equipment, particularly cardiac devices.
Technical Requirements
The Electrically Operated Cardiac Compression Device must meet the following technical specifications:
- Must conform to international quality and performance standards for medical devices.
- Feature adjustable compression rates and effective depth to ensure personalized patient treatment.
- Should be user-friendly, with clear operational guidelines for healthcare providers.
Financial Requirements
Bidders must present a comprehensive financial plan that demonstrates their capability to deliver the required devices within budget constraints. Additional financial criteria include:
- Evidence of sound financial standing and operational capacity.
- Detailed breakdown of pricing, including unit costs and total supply costs, should be outlined clearly.
Document Submission Details
All required documentation must be submitted electronically through the designated e-procurement portal. The submission must include:
- Bidder registration documents
- Technical and financial proposals
- Certificates of compliance and safety standards
Special Provisions
This tender encourages participation from Micro, Small, and Medium Enterprises (MSEs) and recognized startups. Special provisions may include:
- Relaxation in eligibility criteria for MSEs.
- Support and incentives for startups in the medical equipment sector aiming to introduce innovative solutions.
Evaluation Process
The evaluation process for bids will encompass a thorough assessment of technical and financial criteria. Key aspects include:
- Compliance with submission requirements.
- Evaluation of the technical capabilities of the offered devices.
- Competitive pricing and overall value for money.
Delivery Locations
The successful bidder will be required to deliver the Electrically Operated Cardiac Compression Devices to designated healthcare facilities across the jurisdiction of the Department of Health and Family Welfare. Specific locations will be communicated upon awarding the contract.
Contact Information
For any queries or clarifications regarding this tender, bidders may refer to the designated contact point provided in the tender documentation.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Additional Doc 1 (Requested in ATC)
- Additional Doc 2 (Requested in ATC)
- Additional Doc 3 (Requested in ATC)
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity focused on medical devices, possessing valid certifications confirming adherence to health standards, and demonstrating previous supply experience in cardiac devices. Bidders must also showcase their operational stability through financial documents.
The technical specifications include compliance with international medical device standards, adjustable compression settings, and user-friendly interfaces. Devices should also provide adequate depth of compression for varied patient needs, ensuring effective resuscitation.
Bidders must submit certificates that validate their compliance with national health and safety regulations. This includes evidence of quality management systems and adherence to the applicable medical device directives, ensuring that all aspects of the products meet the required health protocols.
The Earnest Money Deposit (EMD) is typically a prerequisite for all bids, ensuring the financial commitment of bidders. The amount required will be specified in the tender documents. Bidders must include this deposit with their submission to qualify for the evaluation process.
Yes, there are significant benefits for MSEs. The tender allows eased eligibility criteria, thereby promoting participation. These provisions aim to support MSEs and startups, encouraging innovation and collaboration within the medical equipment supply sector, consistent with national procurement policies.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders