Tender Timeline
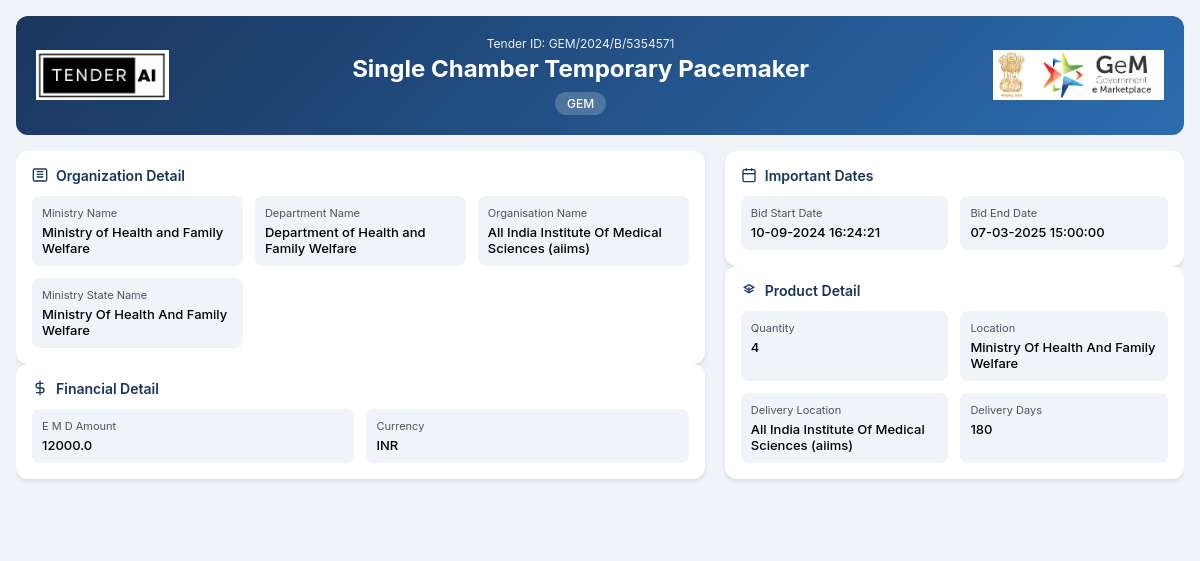
Tender Title: Single Chamber Temporary Pacemaker
Tender Reference Number: GEM/2024/B/5354571
The Single Chamber Temporary Pacemaker tender, issued by the Department of Health and Family Welfare under the Ministry of Health and Family Welfare, aims to procure essential medical equipment to enhance healthcare services. This tender is being released to invite bids from qualified vendors to supply four (4) units of Single Chamber Temporary Pacemakers which are critical in the cardiovascular treatments provided in healthcare facilities.
Scope of Work and Objectives
The aim of this tender is to facilitate the procurement of high-quality Single Chamber Temporary Pacemakers that meet the technical specifications detailed in the accompanying documents. Vendors are encouraged to propose the best solutions that align with the health department’s objectives, emphasizing safety, reliability, and adherence to medical standards.
Eligibility Criteria
To be eligible for this tender, bidders must be registered entities and should possess relevant certifications. They must also comply with the Experience Criteria by providing the necessary documentation that illustrates prior experience in supplying medical equipment. Additionally, if bidders seek exemption from certain experience or turnover criteria, supporting documents must be uploaded for evaluation.
Technical Requirements
Bidders must ensure that the Single Chamber Temporary Pacemakers proposed meet the defined technical specifications outlined in the bidding documents. This includes compliance with all medical standards and quality assurance certifications stated in the Referenced Document provided. Potential bidders are expected to have prior experience in handling similar medical equipment.
Financial Requirements
The Earnest Money Deposit (EMD) for this tender is specified at ₹12,000. Furthermore, bidders should offer a competitive price while demonstrating financial stability to fulfill the contract obligations.
Document Submission Details
All bids must be submitted through the respective portal, including all required documentation such as:
- Experience criteria documentation,
- OEM Authorization Certificates,
- Any other supporting documentation that may establish eligibility.
Documents must conform to the accepted formats specified in the tender documentation.
Special Provisions
There are specific provisions and benefits extended to Micro, Small, and Medium Enterprises (MSEs) and start-ups that apply for this tender. Vendors falling under these categories are encouraged to review these provisions incentivizing participation and can find more details in the linked documents.
Evaluation Process
The evaluation of the bids will be conducted based on a Total Value Wise Evaluation method, prioritizing both technical qualifications and financial evaluations. Successful bidders will be those who offer the lowest price while meeting all specified technical qualifications.
Delivery Locations
The delivery of the medical equipment is expected at designated healthcare facilities as specified by the issuing authority. Details regarding the exact locations can be provided upon request.
Contact Information
For inquiries regarding the tender, interested parties should review the official documentation provided through the linked resources. The main document containing all details regarding the tender specifications, instructions for submission, and contact details can be found here.
Additional Information
Bidders are urged to examine the attached categories, which outline trials allowed as per the approved procurement policy. This will further ensure compliance and readiness for delivering the required medical devices.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Certificate (Requested in ATC)
- OEM Authorization Certificate *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Corrigendum Updates
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity with necessary certifications, having prior experience supplying medical equipment, and providing supporting documentation if requesting exemptions from experience or turnover criteria. It’s crucial to attach all documents during the submission process for evaluation.
Bid submission necessitates several documents, including an OEM Authorization Certificate and proofs detailing prior experience. Suppliers must ensure that their certifications align with the expectations set forth in the tender documents to validate their proposals adequately.
Vendors must register on the relevant government procurement portal to submit their bids. After registration, they’ll be able to access the tender documents, upload necessary certifications, and follow the prescribed guidelines for submission.
Documents must be submitted in the formats specified in the tender guidelines. Typically, PDF or image formats are preferred, but bidders should refer to the official documentation for specific requirements and acceptable versions.
The technical specifications are detailed in the Referenced Document attached in the tender description. Bidders should ensure their offered products comply with all medical standards and requirements outlined therein to qualify for evaluation.
All Single Chamber Temporary Pacemakers must meet the safety and efficacy standards set by the relevant health authorities as well as comply with ISO certifications or equivalent standards specified in the tender documents.
Bidders must demonstrate compliance with all procedural and technical requirements outlined in the tender guidelines, which includes providing detailed experience, the necessary certifications, and adhering to the quality specifications indicated.
Testing criteria will align with established medical device standards, which may include functional testing, compatibility assessments, and safety evaluations, as detailed in the procurement specifications.
The EMD required for this tender is set at ₹12,000. This deposit is necessary to validate bids and will be refunded upon the completion of the bidding process, provided all conditions are met.
Bidders must submit a performance security as specified in the tender documentation, which typically involves submitting a bank guarantee or deposit that ensures contract fulfillment.
Payment terms will be clarified post-awarding the contract. Vendors should review the tender details carefully to understand potential payment schedules and conditions.
Bids will be evaluated based on a Total Value Wise Evaluation, prioritizing vendors who offer the best price while meeting all mandatory technical specifications.
Vendors will receive notifications regarding evaluation results in accordance with the tender submission guidelines. Furthermore, details will typically be shared through the procurement platform utilized for the tender.
Micro, Small, and Medium Enterprises (MSEs) may access special provisions that provide relaxation of certain criteria and additional incentives aimed at promoting participation from smaller enterprises. Specific benefits can be referenced in the available tender documents.
Yes, startups may be entitled to specific benefits and exemptions as per the guidelines outlined in the tender documents, which are designed to encourage innovation and participation in public procurement processes.
This tender aligns with ‘Make in India’ policies by encouraging local manufacturing and procurement solutions that meet the technical needs outlined by the Department of Health and Family Welfare. Compliance with local content requirements is preferred.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders