Department of Health and Family Welfare Tenders (GEM/2025/B/6119344)
Anti Rabies Vaccine (PCEC)
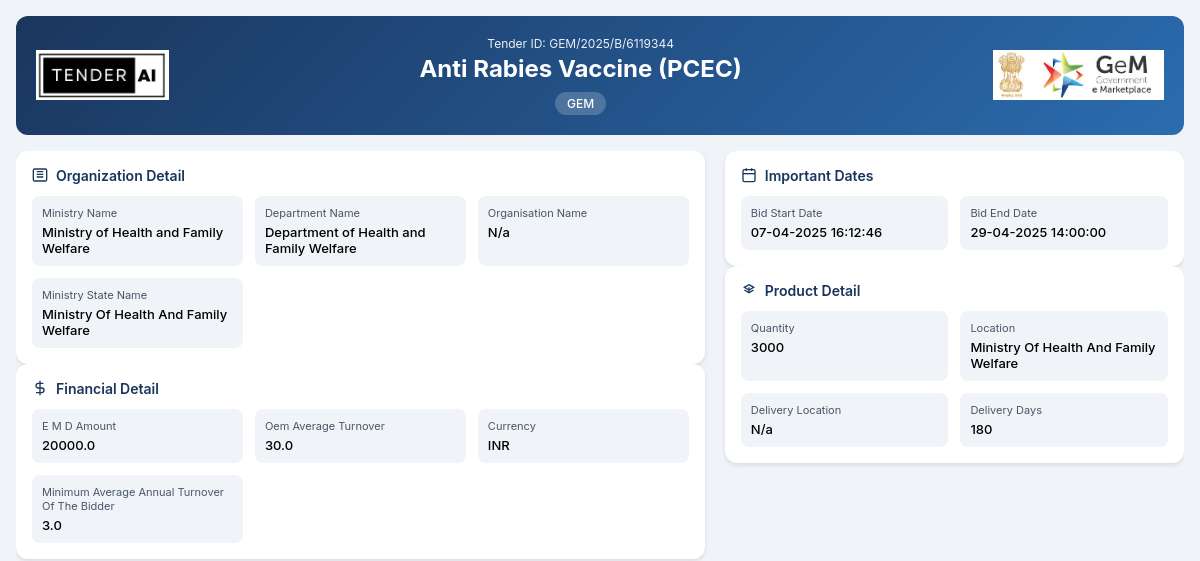
Tender Timeline
Tender Title: Supply of Anti Rabies Vaccine (PCEC)
Tender Reference Number: GEM/2025/B/6119344
Issuing Authority: Department of Health and Family Welfare, Ministry of Health and Family Welfare
The scope of work includes the supply of 3000 packs of Anti Rabies Vaccine (PCEC) to enhance public health initiatives. The objective is to procure an effective rabies vaccine that meets the national health standards to ensure the safety and well-being of the population at risk of rabies exposure.
Eligibility Criteria: Bidders must be registered entities with a valid business license, demonstrating prior experience in supplying pharmaceutical products. Compliance with relevant health regulations and a valid tax registration are essential. Companies must also possess the requisite certifications to distribute medical supplies and pharmaceuticals within India.
Technical Requirements: Suppliers are required to adhere to specified technical standards for the Anti Rabies Vaccine (PCEC). This includes compliance with WHO guidelines and local regulations concerning vaccine efficacy, packaging, and transportation. Documentation verifying production practices, along with quality control measures, needs to be submitted with the proposal.
Financial Requirements: Bidders must provide pricing proposals that reflect competitive market rates, as well as an analysis of the cost-effectiveness of their products. An Earnest Money Deposit (EMD), the amount of which will be specified in the tender documents, is required to ensure commitment to the tender process.
Document Submission Details: Interested parties must submit their technical and financial proposals electronically via the GEM portal. All required documents, including certifications, should be in PDF format for compliance verification.
Special Provisions: Provisions are in place to favor Micro, Small, and Medium Enterprises (MSEs). These benefits will include price preference and exemption from certain eligibility criteria, thus encouraging participation from smaller entities in the supply chain.
Evaluation Process: The evaluation of bids will be conducted based on a two-part process. The technical proposals will first be reviewed for compliance with the specifications outlined in the tender. The financial proposals of qualified technical bids will then be evaluated for cost-effectiveness and value.
Delivery Locations: Vaccines should be delivered to designated health centers across various states as specified in the final contract details post tender evaluation.
Contact Information: For any clarification, prospective bidders may contact the issuing authority’s office, although specific contact details have not been disclosed in this tender document.
This tender presents a significant opportunity to contribute to public health efforts through the supply of essential vaccines. All interested parties are encouraged to participate actively, ensuring compliance with the outlined requirements.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
3 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Additional Doc 1 (Requested in ATC)
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements encompass several criteria to ensure that only qualified vendors participate. Bidders must be registered entities with a valid business license, showcasing their compliance with regulatory standards. They should provide evidence of sufficient experience in supplying similar healthcare products. Additionally, a valid GST or tax registration is essential, alongside necessary certifications for handling and distributing medical supplies. Adherence to industry standards is evaluated to ensure suppliers can meet the quality and delivery timelines required in the tender.
Bidders are required to submit various certifications reflecting their capability to supply the Anti Rabies Vaccine. These include a Good Manufacturing Practices (GMP) certificate, approval from health authorities, and possibly international certifications such as WHO prequalification. Demonstrating compliance with local and international quality standards is critiqued rigorously during the evaluation process to ensure the efficacy and safety of the vaccines.
The Earnest Money Deposit (EMD) is a specified amount that must accompany the bid submission to demonstrate the bidder’s commitment to the tender. This amount will be retained by the issuing authority to ensure compliance with the tender requirements until the contract is awarded. Upon the successful execution of the contract, the EMD may be adjusted against the performance security or refunded. It is imperative to refer to the tender documents for the exact amount and submission guidelines concerning the EMD.
Bidders must submit their proposals electronically through the GEM portal. The accepted document formats primarily include PDF for all official documents, ensuring they are easily accessible for review purposes. Bidders should ensure that all their documents are comprehensively prepared and adhere to the specified submission guidelines in the tender. It is crucial to double-check that all files are properly formatted before submission as per the tender specifications.
Yes, there are special provisions in place for Micro, Small, and Medium Enterprises (MSEs). These benefits include price preferences and exemptions from certain eligibility criteria, aimed at facilitating increased participation from smaller entities. MSEs can also leverage government schemes and incentives that bolster local manufacturing and improve procurement chances while aligning with the ‘Make in India’ initiatives, enhancing their competitiveness in the tendering processes.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders