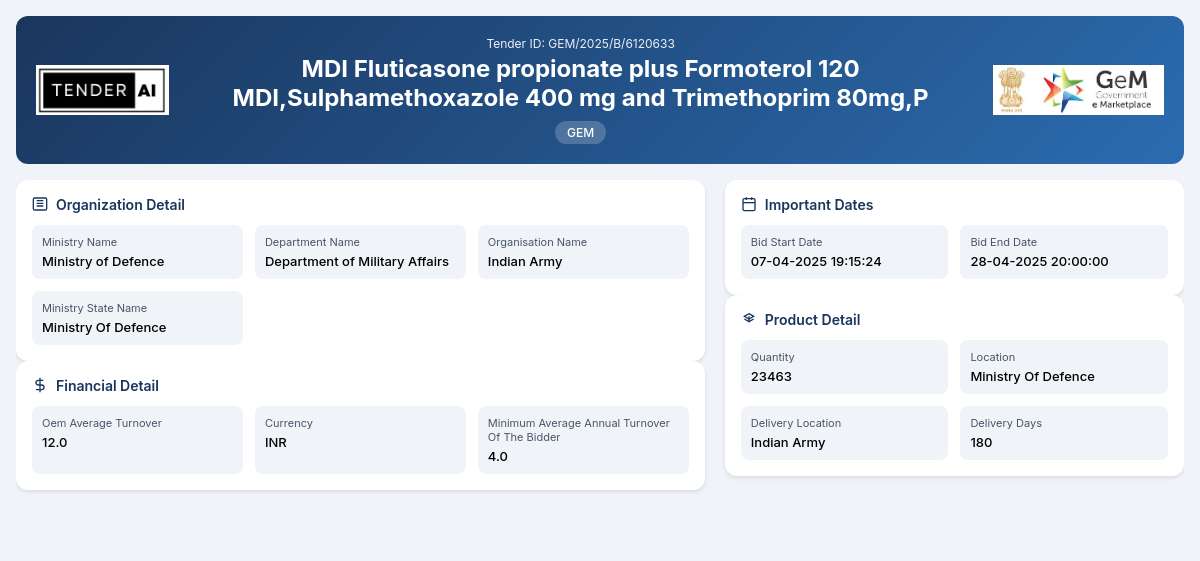
Tender Title: MDI Fluticasone propionate plus Formoterol 120 MDI
Reference Number: GEM/2025/B/6120633
Issuing Authority: Department of Military Affairs, Ministry of Defence
The MDI Fluticasone propionate plus Formoterol 120 MDI tender invites qualified manufacturers to submit their bids for the provision of Fluticasone propionate plus Formoterol in Metered Dose Inhalers (MDI). This pharmaceutical product is critical for treating respiratory conditions and is offered in a total quantity of 23,463 units.
Scope of Work and Objectives
The main objective of this tender is to procure high-quality MDI Fluticasone propionate plus Formoterol to ensure availability for military healthcare facilities. Suppliers are expected to provide a consistent supply of the product while adhering to stringent quality assurance standards.
Eligibility Criteria
Eligible bidders must be registered manufacturers with the capability to fulfill the tender requirements. Compliance with regulatory standards and possessing the necessary certifications are mandatory for participation in this procurement process.
Technical Requirements
The supplied product must meet specific technical specifications, ensuring it aligns with the medical and safety standards applicable in the pharmaceutical field. Documentation proving compliance with quality standards must accompany the bids.
Financial Requirements
Bidders are advised to prepare their financial proposals carefully. Each bid must reflect a competitive pricing structure while ensuring sustainability throughout the procurement lifecycle. Financial stability is essential for consideration.
Document Submission Details
All interested parties must submit the required documents electronically through the specified government e-procurement platform. Complete bid submissions should include technical and financial documentation, aligned with the tender's guidelines.
Special Provisions
Micro, Small, and Medium Enterprises (MSEs) and startups are encouraged to participate, benefitting from provisions designed to enhance their competitiveness in the procurement process. These initiatives aim to bolster local manufacturing under the 'Make in India' campaign.
Evaluation Process
The evaluation process will assess bids based on technical capabilities and value for money. Each submission will undergo a detailed review to ensure compliance with both technical requirements and financial viability.
Delivery Locations
Successful bidders will be required to deliver the goods to designated military healthcare facilities across the country, ensuring timely and efficient distribution of the inhalers.
Contact Information
For more information regarding this tender, parties are encouraged to visit the official Department of Military Affairs procurement portal for further assistance.
This opportunity presents a significant advantage for businesses to engage with a government entity in the healthcare supply domain, contributing to essential services for military personnel.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered manufacturer capable of producing Fluticasone propionate plus Formoterol. Bidders must provide valid licenses and certifications that confirm their compliance with pharmaceutical standards. Additionally, they need to demonstrate financial stability, ensuring they can fulfill large orders and have a history of reliability in similar procurements.
Required certificates for participation in this tender include pharmaceutical manufacturing licenses, quality control certificates, and any other relevant compliance documentation that showcases adherence to regulatory standards in drug manufacturing.
The registration process involves creating an account on the government e-procurement platform where the tender is hosted. Bidders must submit the necessary corporate documents, including company registration, tax compliance certificates, and relevant licenses, before they can access and submit bids for the tender.
The testing criteria for the MDI Fluticasone propionate plus Formoterol focus on efficacy, safety, and stability. All bids must provide proof of quality assurance tests performed in accredited laboratories, ensuring that the products adhere to the highest pharmaceutical standards.
The Earnest Money Deposit (EMD) is required as part of the submission process to ensure commitment from bidders. The specific amount will be defined in the tender documents, and successful bidders may also need to provide a performance security to guarantee the fulfillment of the contract obligations, ensuring that they adhere to the delivery terms and quality expectations specified in the contract.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders