Tender Title and Reference Number
ECHS RE 04 | 202482deb
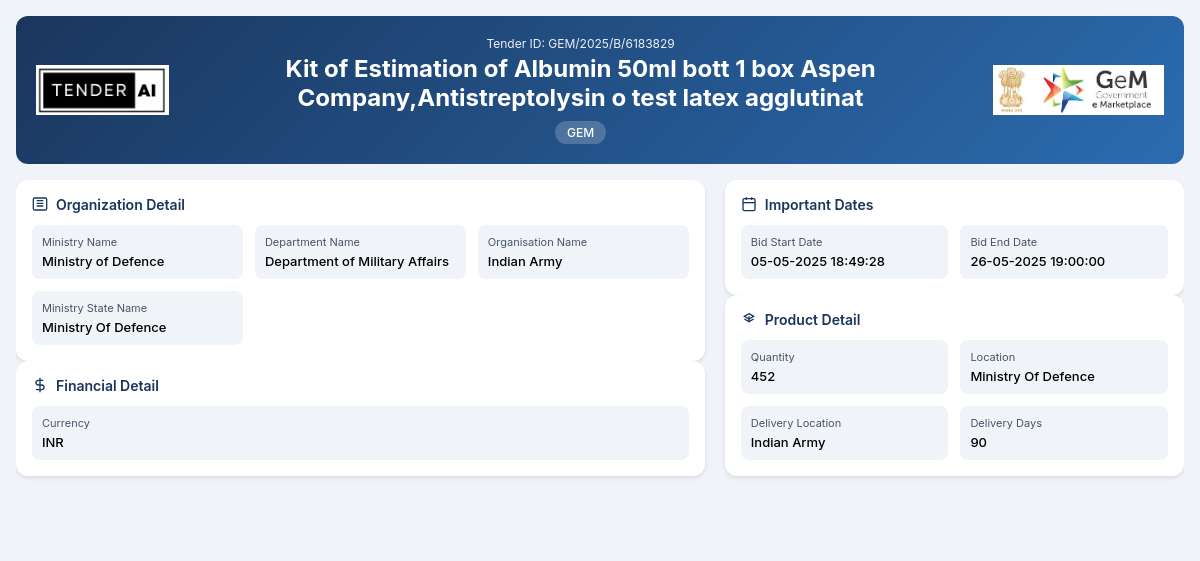
Issuing Authority
Department of Military Affairs, Ministry of Defence
Scope of Work and Objectives
The tender is for the procurement of essential medical testing kits, specifically the Kit for Total Protein Testing and Kit for Triglyceride Estimation. These kits consist of Albumin 50ml Bottles and Antistreptolysin O Test Latex Agglutinat. The total quantity required for this procurement is 452 kits. The main objective is to ensure a robust supply of reliable medical test kits that can be utilized effectively within military healthcare facilities.
Eligibility Criteria
Potential bidders must meet specific eligibility requirements to participate in this tender:
- Be a registered entity authorized to supply medical equipment.
- Have previously supplied similar products or have relevant expertise in the medical procurement sector.
- Ensure compliance with all applicable regulations and standards issued by health authorities.
Technical Requirements
Bidders are expected to adhere to stringent technical specifications for the medical kits. The products must:
- Meet international quality standards pertaining to medical supply.
- Be delivered as sealed, shelf-stable products with necessary certifications.
- Include a comprehensive manual or specification sheet indicating usage and safety standards.
Financial Requirements
Bidders should demonstrate financial capability by providing relevant documents including:
- Audited financial statements for the last three fiscal years.
- Evidence of having executed contracts of similar value.
- No outstanding dues to the government from previous contracts.
Document Submission Details
Interested suppliers must submit the following documents:
- Technical and financial proposals.
- Certificates of registration and compliance with health and safety standards.
- A duly filled tender submission form as outlined in the tender document.
Special Provisions
There are provisions available for Micro, Small, and Medium Enterprises (MSEs) and start-ups that encourage their participation in this tender. Eligible companies may benefit from reduced qualification criteria and could also enjoy reserved quotas.
Evaluation Process
The evaluation process will involve:
- Checking the eligibility requirements based on the submitted documents.
- Technical evaluation of the proposals focusing on product quality and compliance.
- Financial assessment to ensure that bids are within budget and offer good value.
Delivery Locations
The delivery locations specified will likely include military hospitals and health facilities under the jurisdiction of the Department of Military Affairs.
Contact Information
For any queries regarding submission and requirements, potential bidders are encouraged to reach out to the Department of Military Affairs through the official communication channels provided in the tender document.
Tender BOQ Items
41 ItemsGeneral Information
Financial Information
Evaluation and Technical Information
Tender Documents
5 DocumentsSimilar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity capable of supplying medical testing kits, demonstrating prior experience in similar procurements, and complying with all applicable health and safety regulations. Potential bidders must provide documentation supporting their registration and previous successful deliveries of medical supplies.
Bidders are required to provide a range of certificates including:
- Compliance certificates from health authorities.
- GST registration documents.
- Quality assurance certifications related to medical equipment. These documents are crucial for assessing the quality and safety standards of the products being supplied.
The registration process involves submitting an expression of interest through the designated portal. Interested suppliers must ensure that they are registered to participate in GEM (Government e-Marketplace) procurements. Detailed guidelines are usually provided within the tender document, including the steps for submitting necessary documentation.
All submitted documents must be in electronic format, typically PDF or Microsoft Word. Any additional forms or templates specified in the tender document should be filled accurately. Bidders must ensure adherence to any formats prescribed, as non-compliance may lead to disqualification.
Yes, there are benefits for MSEs and start-ups designed to encourage their participation in government tenders. Such benefits may include relaxed eligibility criteria, dedicated top quota set-asides, and financial support schemes, contributing positively to the growth and competitiveness of small businesses in the medical equipment sector.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders