Digital Medical X - Ray Films (V2)
Digital Medical X - Ray Films (V2)
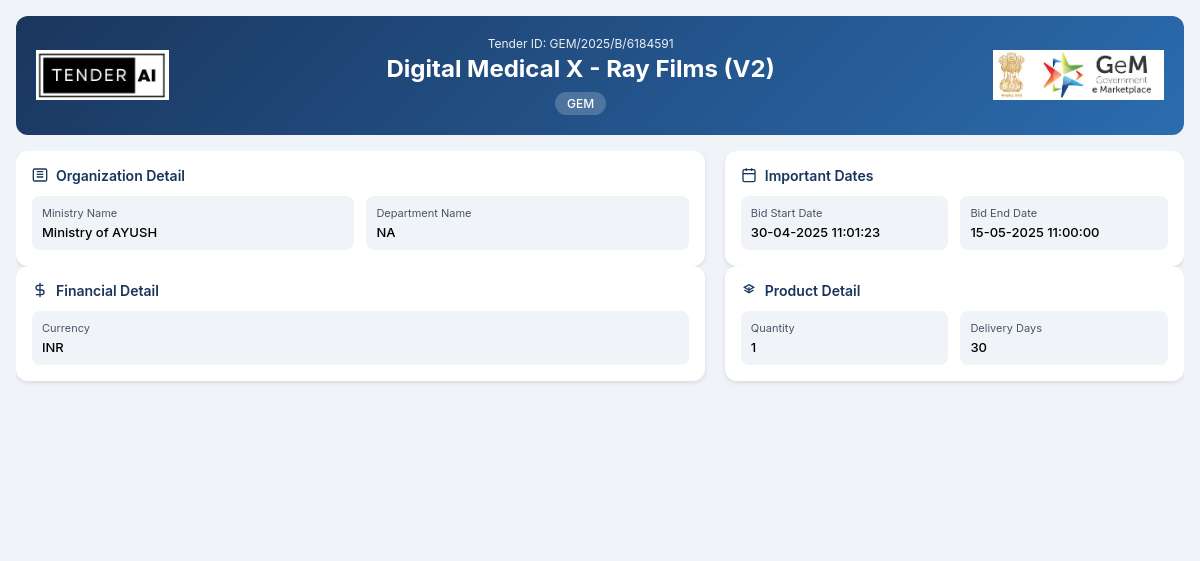
Tender Timeline
Tender Title: Digital Medical X - Ray Films (V2)
Reference Number: 23d0271c3
Issuing Authority: Ministry of AYUSH
The Ministry of AYUSH invites bids for the supply of Digital Medical X - Ray Films (V2), aimed at enhancing the healthcare delivery system within various medical facilities. This tender represents an integral part of the government's initiative to improve diagnostic services across hospitals, clinics, and healthcare centers nationwide.
Scope of Work and Objectives
The scope includes supplying 1,500 units of high-quality Digital Medical X - Ray Films as per the prescribed specifications and requirements. The primary objective is to ensure that healthcare providers have access to advanced diagnostic tools, thus facilitating better patient care and treatment outcomes. The successful bidder will be responsible for the delivery, installation, and any necessary training associated with the use of these films.
Eligibility Criteria
To qualify for this tender, bidders must meet specific eligibility requirements, including but not limited to:
- Being a registered entity in the relevant business domain.
- Demonstrating past experience in supplying medical imaging equipment or consumables.
- Compliance with all regulatory requirements governing medical devices.
Technical Requirements
The Digital Medical X - Ray Films must meet the following technical specifications:
- Compatibility with standard digital X-ray machines.
- High resolution and clarity for diagnostic purposes.
- Appropriate storage conditions to maintain integrity and performance.
Financial Requirements
Bidders must provide a comprehensive quotation reflecting all costs associated with the supply of the films. This includes pricing per unit, any applicable taxes, and potential delivery charges. Financial transparency is crucial and encourages fair evaluation.
Document Submission Details
Bidders are required to submit their proposals electronically, ensuring that all documentation is complete and adheres to the prescribed format laid out in the tender guidelines. Submitted documents must encompass:
- Technical proposal
- Financial bid
- Valid certifications and registration documents
- Compliance with any other specified requirements
Special Provisions
This tender encourages participation from Micro, Small, and Medium Enterprises (MSEs) by providing certain benefits, facilitating inclusivity and support for startups. The provisions may include priority during the evaluation process and relaxed eligibility criteria.
Evaluation Process
The evaluation of tenders will be conducted based on predefined criteria that include technical capability, pricing, delivery timelines, and compliance with specifications. The submission will undergo a multi-stage evaluation to ensure thorough scrutiny and fair selection of the winning bidder.
Delivery Locations
Successful bidders will be notified of delivery locations upon the awarding of the contract. Locations may vary based on hospital distribution and healthcare needs.
Contact Information
Specific contact information for inquiries is acknowledged in the tender documentation. Bidders are encouraged to consult this for any clarifications regarding the tender.
The Ministry of AYUSH looks forward to receiving competitive bids from all interested parties and aims to establish partnerships that enhance healthcare facilities in the region.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
3 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Corrigendum Updates
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity, demonstrating relevant experience in supplying medical imaging tools, and complying with all regulatory laws governing medical devices. Additionally, bidders must meet any preliminary criteria as specified in the tender documentation.
The Digital Medical X - Ray Films must provide high resolution and clarity, ensuring compatibility with standard diagnostic equipment. Films should maintain their integrity under specified storage conditions to guarantee optimal performance for medical purposes.
Bidders are typically required to submit an EMD as part of their proposal to demonstrate their commitment and financial capability. The specific amount and conditions related to the EMD will be outlined in the tender documentation, ensuring compliance with the bidding rules.
Bids must be submitted electronically in the required document format specified in the tender guidelines. Accepted formats generally include PDF and Word documents, ensuring the integrity of the bid without alterations during the submission process.
Yes, there are specific provisions that support Micro, Small, and Medium Enterprises (MSEs). These may include a more lenient evaluation process, priority in bidding, and potential financial incentives aimed at fostering entrepreneurship and innovation within the sector.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders