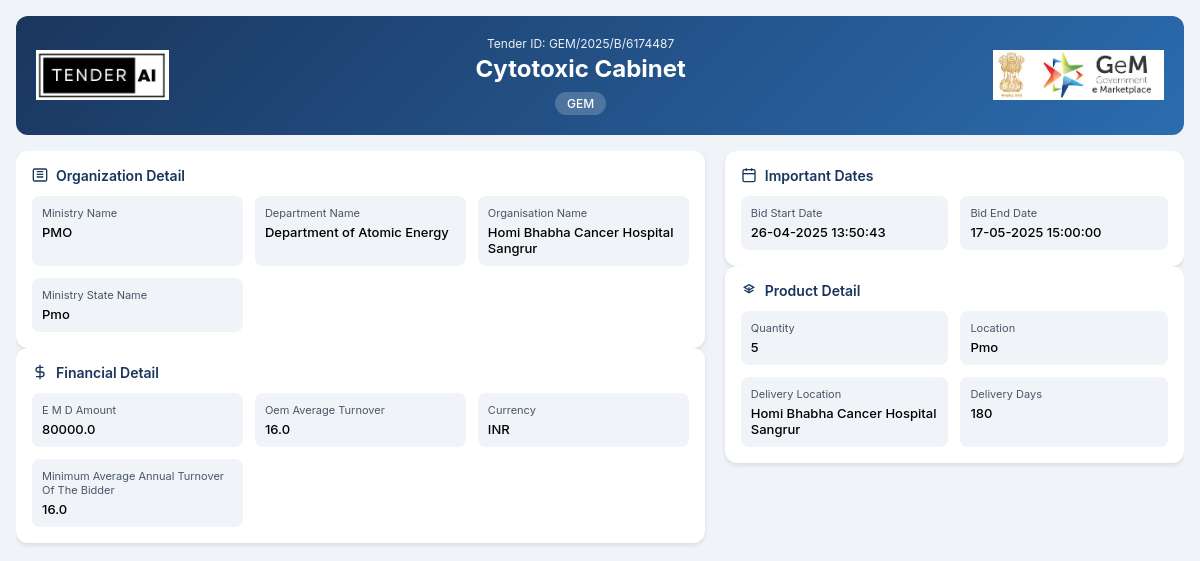
Tender Title: Cytotoxic Cabinet
Tender Reference Number: GEM/2025/B/6174487
Issuing Authority/Department: Department of Atomic Energy
The Cytotoxic Cabinet tender, reference number GEM/2025/B/6174487, aims to procure specialized equipment essential for handling hazardous cytotoxic substances. The successful vendor will be required to supply up to five (5) cytotoxic cabinets, ensuring compliance with stringent safety and operational standards to guarantee the protection of personnel and the environment.
Scope of Work and Objectives
The primary objectives of this tender include:
- Supply of cytotoxic cabinets designed for safe handling of hazardous materials.
- Ensuring that all equipment meets the required technical specifications and safety standards.
- Provide installation, training, and maintenance services, if necessary.
Eligibility Criteria
To participate in this tender, bidders must qualify based on the following eligibility criteria:
- Must be a registered entity with valid operating licenses.
- Possess relevant experience in supplying similar healthcare equipment.
- Proven track record for timely delivery and quality compliance.
Technical Requirements
Bidders are expected to adhere to specific technical requirements which include:
- Certification of compliance with international safety standards for cytotoxic handling.
- Documentation validating the functionality and efficiency of the equipment.
- Provision of user manuals and warranty specifics.
Financial Requirements
Bidders must provide detailed financial documentation, including:
- A breakdown of costs for the equipment.
- Proof of ability to finance the procurement.
- Submission of Earnest Money Deposit (EMD) as per tender specifications.
Document Submission Details
All submissions must be made electronically, following the guidelines laid out in the tender documentation. Necessary documents include eligibility proof, technical specifications, and financial records, to ensure smooth evaluation.
Special Provisions
The tender encourages participation from Micro, Small, and Medium Enterprises (MSEs). Provisions for startups allowing for support under government initiatives are also included, enhancing competitiveness in the bidding process.
Evaluation Process
The evaluation of bids will be conducted systematically based on:
- Compliance with eligibility and technical specifications.
- Financial viability and pricing structure. Results and notifications will be communicated following the evaluation process.
Delivery Locations
The delivery of cytotoxic cabinets will be directed to specified locations as outlined in the tender documentation. Bidders should ensure their capabilities to meet the logistical requirements for timely delivery.
Contact Information
For any inquiries regarding this tender, bidders are encouraged to reach out to the Department of Atomic Energy through official channels mentioned in the tender documentation.
This tender presents an opportunity for qualified suppliers to contribute to critical healthcare safety initiatives through the provision of specialized cytotoxic cabinets. Interested parties should prepare their submissions in accordance with the outlined criteria and ensure adherence to technical and financial requirements for consideration.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Additional Doc 1 (Requested in ATC)
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity with valid operating licenses. Bidders must also demonstrate relevant experience in supplying similar healthcare equipment and provide evidence of a proven track record for timely delivery and quality compliance. Meeting these criteria is essential to participate in the tender.
To qualify, bidders must submit specific certificates that validate compliance with international safety standards, particularly regarding cytotoxic handling protocols. Additionally, bidders are required to provide proof of relevant industry certifications that demonstrate technical capability and experience with similar products.
The registration process requires vendors to complete an application as outlined in the tender documentation. Participants must ensure that they meet all qualification requirements and submit appropriate documents that fulfill eligibility criteria, including registration details, technical certifications, and financial statements.
Bidders will be required to provide a performance security, typically a predetermined percentage of the total bid amount, to ensure compliance with the contract terms and conditions. This security must be furnished before contract execution and is essential for safeguarding interests in case of non-fulfillment of obligations.
The evaluation and selection process will include rigorous assessments based on technical compliance with specifications outlined in the tender, financial evaluation relating to pricing strategies, and overall value propositions. Notifications regarding the results will follow the completion of the evaluation process, ensuring transparency and fairness.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders