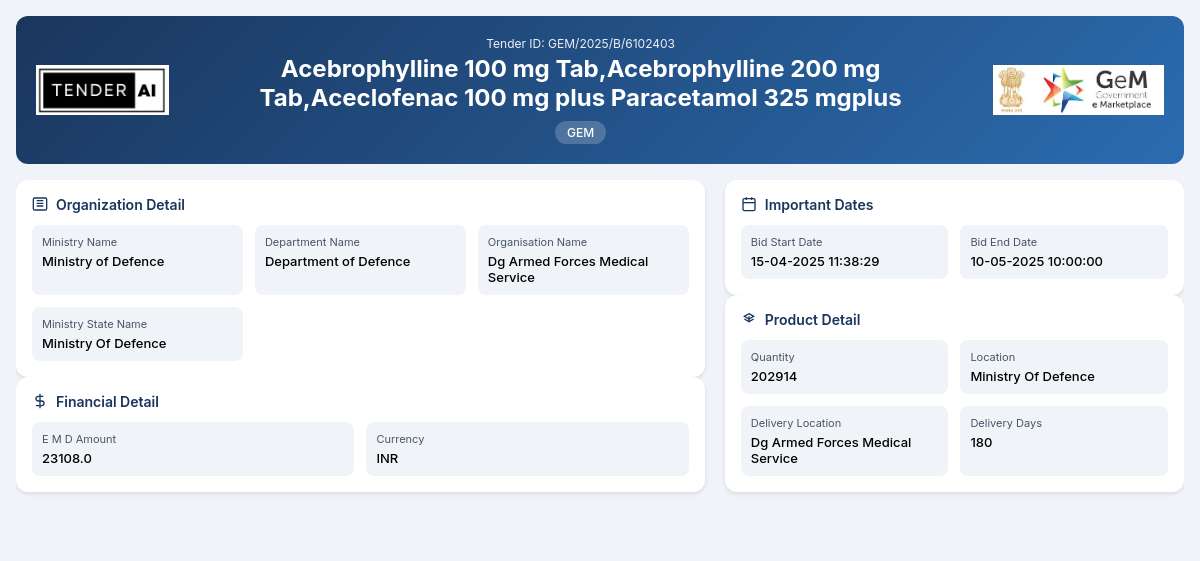
Tender Title and Reference Number
Title: Supply of Pharmaceuticals
Reference Number: GEM/2025/B/6102403
Issuing Authority/Department
The tender is issued by the Department of Defence, under the Ministry of Defence. This prestigious government body is responsible for ensuring that the defence services have adequate and timely supplies of essential pharmaceuticals, contributing significantly to the well-being of personnel.
Scope of Work and Objectives
The primary objective of this tender is to procure a wide range of pharmaceutical products, including but not limited to Acebrophylline tablets, Aceclofenac combined with Paracetamol, and various other essential medications. The total quantity being sourced is approximately 202,914 units, aimed at addressing the medical needs within the defence sector. Suppliers are expected to fulfill the requirements efficiently and maintain the highest standards of quality and compliance with prescribed regulations.
Eligibility Criteria
To qualify for this tender, bidders must comply with specific eligibility criteria. This includes being a registered entity with the relevant governing bodies, possessing the necessary licenses for pharmaceutical distribution, and having experience in dealing with government or defense contracts. Companies must demonstrate their capability to manage large volume orders while adhering to the time frames and specifications stipulated within the tender documents.
Technical Requirements
The technical requirements for this tender encompass compliance with established pharmaceutical quality standards, including certifications for Good Manufacturing Practices (GMP). All drugs must meet safety regulations and include detailed product specifications, including active ingredients and dosages, to ensure efficacy and safety for military personnel.
Financial Requirements
Bidders must provide detailed financial documentation, including proof of solvency to support their ability to meet the scope of work financially. Payment terms will be outlined within the contractual agreement, with adherence to government procurement policies for timely settlements.
Document Submission Details
All bids must be submitted through the online tendering platform, ensuring that electronic documents are in acceptable formats (PDF, DOCX) without exceeding stipulated size limits. Bidders should ensure their submissions are complete by including requisite certifications and supporting documents.
Special Provisions
The tender supports Micro, Small, and Medium Enterprises (MSEs) by providing opportunities for participation, which align with government initiatives to boost local suppliers and startups. Provisions for startups may also include relaxed eligibility criteria, promoting a diverse range of competitors.
Evaluation Process
The evaluation process will assess bids based on both technical criteria and financial aspects. Factors such as quality assurance procedures, past performance, and delivery timelines will contribute to the overall evaluation score. A transparent and fair process will ensure that the most capable suppliers are selected.
Delivery Locations
Deliveries must be executed to specified addresses within the jurisdiction of the Department of Defence. Bidders are responsible for providing accurate delivery schedules and ensuring compliance with delivery requirements.
Contact Information
For further inquiries, bidders are encouraged to reach out through the designated contact channels listed on the tender platform. It’s vital to ensure all communications are documented for transparency.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Additional Doc 1 (Requested in ATC) *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity that possesses the necessary licenses for pharmaceutical distribution and having a proven track record in handling government contracts. Bidders should demonstrate their capability to manage large orders while ensuring compliance with quality standards for vital pharmaceuticals.
Bidders must ensure their products comply with established technical specifications such as Good Manufacturing Practices (GMP) and necessary safety certifications. Specifications of active ingredients, dosages, and any additional attributes relevant to quality standards must be included in bid submissions.
Payment terms will be stipulated within the final contract, typically involving payments against delivery and acceptance of goods. An earnest money deposit or performance security may be required to ensure compliance with contract terms, aimed at protecting the interests of the Department of Defence.
Submissions must be made electronically via the government’s online tendering platform. Accepted document formats include PDF and DOCX. Bidders should ensure that their submissions meet the size and format limitations specified in the tender documentation to avoid disqualification.
This tender provides unique benefits for Micro, Small, and Medium Enterprises (MSEs), as well as startups, by allowing them to participate under preferential terms. Such provisions may include simplified registration processes and support in meeting compliance with ‘Make in India’ policies, encouraging local content and procurement rules.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders