Department of Defence Tender by Dg Armed Forces Medical Service (GEM/2025/B/6079033)
Metronidazole Inj for IV use containing 500 mg per bott of 100 ml
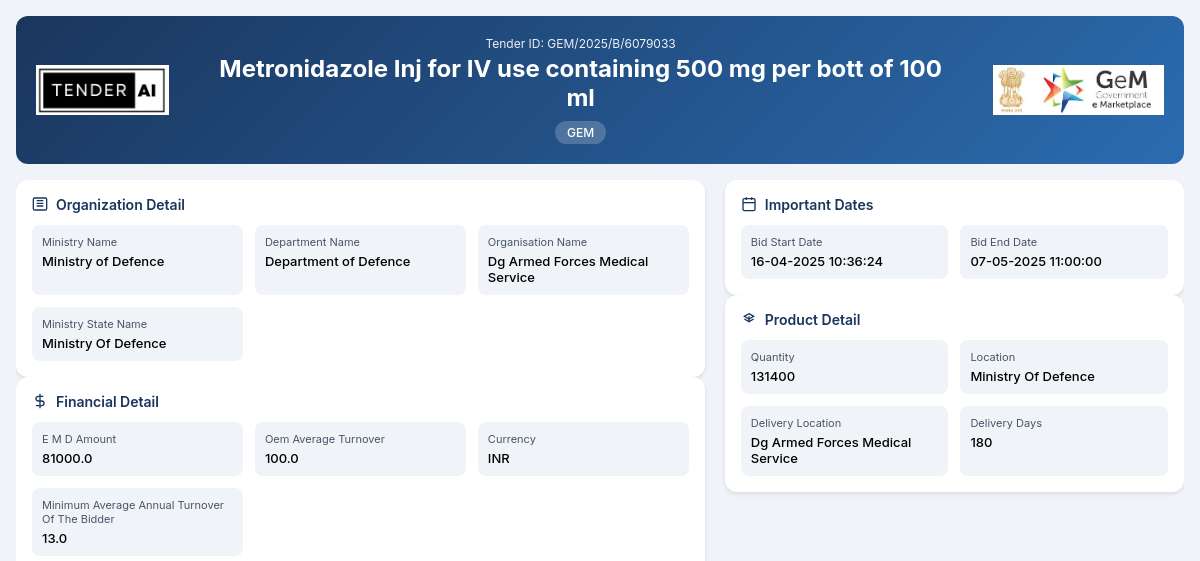
Tender Timeline
Tender Title: Metronidazole Inj for IV use
Tender Reference Number: GEM/2025/B/6079033
Issuing Authority: Department of Defence, Ministry of Defence
Scope of Work and Objectives
This tender focuses on the procurement of Metronidazole Injection for intravenous (IV) use, specifically formulated to contain 500 mg per bottle of 100 ml. The objective is to secure a reliable supply of this essential pharmaceutical product to meet the needs of healthcare facilities operated under the Ministry of Defence.
Eligibility Criteria
To qualify for this tender, bidders must be duly registered entities, compliant with all applicable laws and regulations. Additionally, bidders should have experience in the procurement and supply of pharmaceutical products, ensuring adherence to established quality standards and regulatory guidelines.
Technical Requirements
Bidders are required to provide Metronidazole injection that meets the specified technical requirements, including:
- Comprises 500 mg of Metronidazole per 100 ml bottle.
- Must meet all safety and efficacy benchmarks as prescribed by the health regulatory authorities.
- Complete documentation proving compliance with health and safety standards, including evidence of manufacturing processes.
Financial Requirements
Bidders must showcase their financial stability, providing documents including but not limited to:
- Financial statements for the last two financial years.
- Evidence of compliance with applicable financial regulations.
Document Submission Details
All proposals must be submitted through the designated online portal. Bidders should ensure that each document uploaded is complete, clearly labeled, and adheres to the specified formats as outlined in the tender documentation.
Special Provisions
This tender promotes inclusivity by encouraging participation from Micro, Small, and Medium Enterprises (MSEs). Benefits for MSEs include possible concessional tender evaluation criteria and easier access to procurement opportunities.
Evaluation Process
Bids will undergo a comprehensive evaluation process based on predefined parameters that include:
- Compliance with the technical specifications laid out in the tender.
- Financial viability and pricing offered by bidders.
- Quality credentials and past performance metrics.
Delivery Locations
Successful bidders will be required to deliver the products to designated healthcare facilities as specified by the Ministry of Defence. Delivery conditions and schedules will be finalized upon contract award.
Contact Information
For queries related to the tender, bidders can refer to the official Department of Defence contact channels provided in the document.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Additional Doc 1 (Requested in ATC)
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity with a valid business license. Bidders must demonstrate their capability in supplying pharmaceutical products and comply with all relevant regulations. They should possess the necessary technical knowledge and prior experience in similar contracts to qualify for this tender.
Bidders must ensure that the Metronidazole Injection meets stringent technical specifications that include dosage accuracy (500 mg per 100 ml), compliance with safety standards, and certification by health authorities regarding manufacturing processes. Documentation proving adherence to these specifications must be submitted with the bid.
Bidders are generally expected to provide an Earnest Money Deposit (EMD) as a part of the tender submission, which will be specified in the tender documentation. Upon winning the contract, bidders must also present a performance security that guarantees fulfillment of contractual obligations, ensuring that quality standards are upheld throughout the project duration.
Documents must be submitted via the designated online submission platform. It is imperative that all required documents adhere to specified formats, including PDF and other accepted formats, to ensure compatibility with the tender review process. Proper labeling of each document facilitates efficient evaluation.
Micro, Small, and Medium Enterprises have access to special provisions which may include relaxed eligibility criteria, supportive guidelines for participation, and potential preferential treatment during bid evaluations. This encourages broader participation and supports the growth of smaller businesses within the procurement landscape.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders