Department of Health and Family Welfare Tender by Directorate Of Health Services Kashmir (GEM/2024/B/5594156)
Single Chamber Pacemaker 1,Single Chamber Pacemaker 2,Single Chamber Pacemaker 3,Dual chamber Pacem
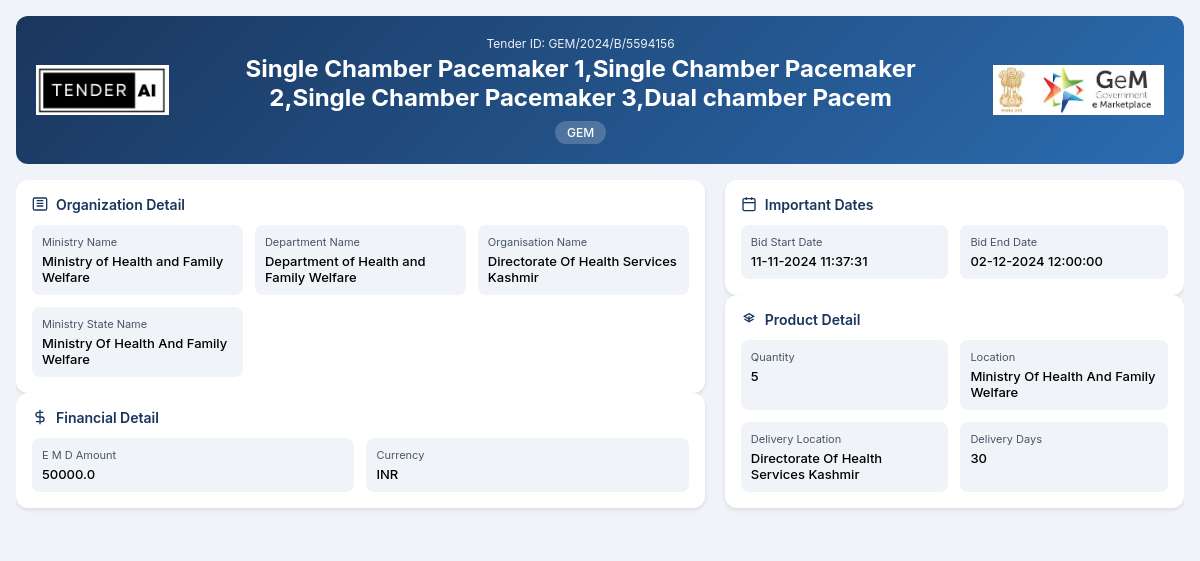
Tender Timeline
Tender Title: Supply of Single and Dual Chamber Pacemakers
Reference Number: GEM/2024/B/5594156
Issuing Authority/Department:
The tender is issued by the Department of Health and Family Welfare, under the Ministry of Health and Family Welfare. The procurement is conducted by the Directorate Of Health Services Kashmir.
Scope of Work and Objectives:
This tender aims to acquire a total quantity of five pacemakers, which include multiple categories such as Single Chamber Pacemaker 1, Single Chamber Pacemaker 2, Single Chamber Pacemaker 3, Dual Chamber Pacemaker 4, and Dual Chamber Pacemaker 5. The primary objective is to enhance cardiac care by supplying reliable and high-quality medical devices that meet stringent specifications laid down by health authorities. Providers will be expected to demonstrate both technical capacity and regulatory compliance to secure the contract.
Eligibility Criteria:
Bidders must satisfy the following eligibility criteria:
- Must be a registered entity in the related field.
- Possess a minimum of 3 years of past experience in supplying similar medical devices.
- Provide evidence of past performance with a minimum required score of 70%.
- Submit OEM Authorization Certificates and other supporting documents as detailed in the tender documentation.
Technical Requirements:
Bidders are required to meet the following technical specifications:
- Compliance with BoQ specifications.
- Submission of appropriate testing and quality certificate documents as needed for evaluation.
- Delivery of equipment that meets or exceeds safety and quality standards prescribed by medical equipment regulations.
Financial Requirements:
Bidders should also be aware of the financial obligations:
- An Earnest Money Deposit (EMD) of INR 50,000 is required.
- The submission of a performance security as stipulated in the tender documentation will be necessary upon contract award.
Document Submission Details:
Bidders must prepare and submit a comprehensive bid documentation package, which includes:
- Experience Criteria and Past Performance.
- Bidder Turnover for the past three years.
- Certificates requested in the Additional Terms and Conditions (ATC).
- OEM Annual Turnover and Compliance documents.
- In the case of seeking exemptions from Experience/Turnover Criteria, appropriate supporting documents must be uploaded for evaluation.
Special Provisions:
This tender includes benefits for Micro, Small, and Medium Enterprises (MSEs) and start-ups, offering them preferential treatment during the evaluation process. Suppliers indicating their status as an MSE or startup must provide necessary documentation as evidence.
Evaluation Process:
The evaluation method adopted will focus on a Total Value Wise Evaluation, taking into account both the price and compliance with the specifications. Each submission will be assessed based on the criteria outlined, ensuring a fair and transparent selection of the best supplier.
Delivery Locations:
Successful bidders will be required to deliver the specified medical devices to predetermined locations based on the Department's requirements, which will be clarified post-award.
Contact Information:
For further queries or clarification, bidders are encouraged to reach out via the links provided in the main document, as direct contact information is not explicitly stated.
Additional Information:
- Bidders should be prepared to adhere to the 'Make in India' policy where applicable.
- The submission method for bids will be via electronic upload through the designated platform.
- The method and timeline for bid opening and evaluation are clearly defined in the tender document.
For complete details and documentation, bidders can download the main document here.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
6 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Corrigendum Updates
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity, having a minimum of 3 years of relevant experience in supplying medical devices, and demonstrating a minimum score of 70% in past performance. Additionally, bidders must submit OEM authorization and compliance documentation as specified.
Bidders must provide essential certificates including OEM Authorization Certificates, compliance with BoQ specifications, and proof of past performance and turnover for the past three years. Detailed criteria are outlined in the Additional Terms and Conditions (ATC) of the tender.
To register for bidding, vendors must create an account on the Gem Portal, complete the necessary registration process by providing required business documentation, and subsequently follow the instructions for bidding outlined in the tender document.
Accepted document formats typically include PDF and other commonly used file formats. It’s essential for bidders to comply with the specified format to ensure successful submission of their application.
Yes, bidders need to comply with the technical specifications stipulated in the BoQ, which include strict adherence to quality and safety standards for medical devices, as stated in the tender documentation.
All supplied equipment must meet approved regulatory quality standards as defined by applicable health regulations. Bidders are encouraged to provide evidence of certifications and compliance in their submissions.
Bidders must ensure compliance with health and safety regulations, provide OEM certifications, and confirm adherence to the ‘Make in India’ policies where applicable.
The testing criteria will involve evaluations based on the manufacturer’s specifications and compliance with safety standards, including performance testing as required by the Department of Health.
The Earnest Money Deposit (EMD) is set at INR 50,000 and must be submitted to secure the bid. This amount will be refunded to non-winning bidders post-evaluation as per the tender regulations.
Upon award, bidders are required to submit performance security, the amount and conditions of which will be detailed in the contract documentation provided following the award of the tender.
Payment terms typically involve staggered disbursements based on delivery and acceptance of the products. Detailed payment milestones will be outlined in the contract upon successful bidding.
Price evaluations will consider not only the quoted price but also compliance with specifications, delivery timelines, and vendor qualifications as part of a well-rounded evaluation process.
Bids must be submitted electronically through the Gem Portal, ensuring that all required documents are uploaded in the specified formats outlined in the tender guidelines.
The evaluation and selection process is thorough, focusing on both technical compliance and price. Bids will be assessed based on value for money and adherence to specified criteria, as outlined in the tender.
Yes, all bidders will be notified of the selection results through the Gem Portal, ensuring transparency in the bidding process. Subsequent actions and communications will follow based on the results announced.
Yes, there are specific benefits for Micro, Small, and Medium Enterprises (MSEs), including preferential evaluation and provisions in the selection process, allowing MSEs to compete effectively.
Start-ups can avail of certain provisions and incentives during the tender evaluation process, as outlined in the public procurement policies, fostering inclusivity for new entrants in the industry.
This tender supports the ‘Make in India’ initiative by encouraging local manufacturing and procurement practices, thereby boosting domestic capabilities in the health sector. Bidders are encouraged to confirm alignment with local sourcing requirements.
Local content requirements may influence procurement decisions, encouraging bidders to indicate their percentage of local sourcing in compliance with national policies aimed at fostering domestic industries.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders