Department of Health and Family Welfare Tender by Hll Infra Tech Services Limited (GEM/2025/B/6065591)
Dual Pump Transversal Phaco Machine for Micro-Incision Cataract Surgery - High End
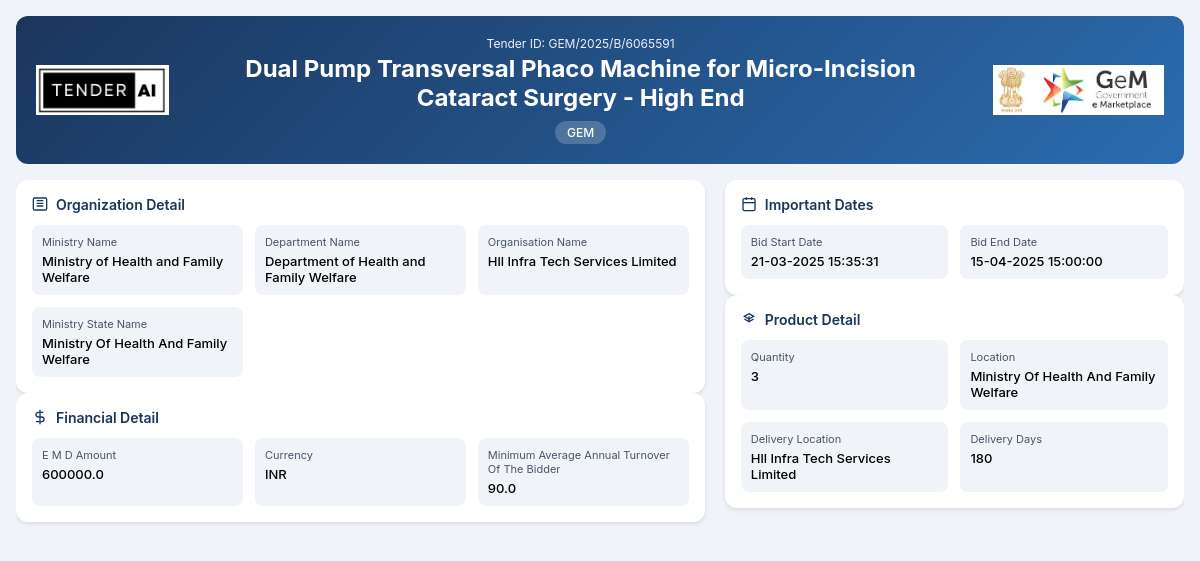
Tender Timeline
Tender Title: Dual Pump Transversal Phaco Machine for Micro-Incision Cataract Surgery - High End
Tender Reference Number: GEM/2025/B/6065591
Issuing Authority/Department: Department of Health and Family Welfare, Ministry of Health and Family Welfare
The Dual Pump Transversal Phaco Machine for Micro-Incision Cataract Surgery - High End tender aims to procure advanced medical equipment crucial for enhancing surgical procedures in the healthcare domain. This high-end phacoemulsification system is designed to improve outcomes in micro-incision cataract surgeries, known for their precision and reduced recovery times.
Scope of Work and Objectives
The primary objective of this tender is to acquire three units of the dual pump transversal phaco machine which will be used in various healthcare settings across the nation. The equipment must meet international standards and specifications to ensure efficacy during cataract surgeries. Suppliers are required to present comprehensive proposals that outline the capability, durability, and technical superiority of their machines.
Eligibility Criteria
To qualify for participation, bidders must be registered entities under relevant laws and possess proven experience in supplying medical equipment. Bidders are also encouraged to demonstrate compliance with necessary health regulations and certifications stipulated by the Department of Health and Family Welfare.
Technical Requirements
Proposed phaco machines should meet the following technical specifications:
- Dual Pump Technology
- Advanced phacoemulsification features
- Compatibility with micro-incision techniques
- User-friendly interface
- High reliability and robustness in performance
Additionally, all equipment should be ISO certified and comply with Indian Medical Device regulations.
Financial Requirements
Bidders must demonstrate financial stability by providing financial statements for the past three years. This includes ensuring that the offered pricing is competitive and aligned with the current market conditions for similar high-end medical devices.
Document Submission Details
Bidders need to submit detailed proposals which include technical specifications, compliance documentation, financial statements, and terms of warranty and support. All documentation should be made available in electronic format, in line with early specifications released during the tender announcement.
Special Provisions
Startups and Micro, Small, and Medium Enterprises (MSEs) are especially encouraged to bid. These entities might benefit from relaxed eligibility parameters and special considerations in the evaluation process to promote inclusivity in government procurement practices.
Evaluation Process
The evaluation will focus on both technical and financial aspects. Technical proposals will be assessed for compliance with specifications, innovative features, and past performance. Financial proposals will be scrutinized for pricing competitiveness and value for money. The tender evaluation committee will shortlist bidders based on a scoring system designed to balance quality and cost.
Delivery Locations
The successful bidders will be required to deliver the equipment to designated healthcare facilities as identified in the procurement strategy by the Department of Health and Family Welfare.
Contact Information
While specific contact details are not provided, all queries related to the tender can be directed through the official channels of the Ministry of Health and Family Welfare. Solutions for technical and procedural inquiries are typically addressed through the official procurement portals.
This tender represents an important step toward enhancing surgical capabilities in India, ensuring that healthcare providers are equipped with the best available technologies for micro-incision cataract surgeries. Interested participants are encouraged to prepare their bids meticulously, adhering to all specified requirements to ensure a seamless evaluation process.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- Additional Doc 1 (Requested in ATC)
- Additional Doc 2 (Requested in ATC)
- Additional Doc 3 (Requested in ATC)
- Additional Doc 4 (Requested in ATC)
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Corrigendum Updates
Similar Tenders
Frequently Asked Questions
The eligibility requirements stipulate that bidders must be registered entities recognized under Indian law. Additionally, they must demonstrate previous experience in supplying similar medical equipment and compliance with health regulations set forth by the Department of Health and Family Welfare.
Bidders are required to furnish certifications of compliance with relevant Indian Medical Device Regulations and ISO certifications as proof of quality standards. This ensures the machine’s reliability and effectiveness in clinical settings, particularly during micro-incision cataract surgeries.
The registration process involves submitting the intended documentation electronically through the government procurement portal. It is crucial to prepare and verify all required documents, including proof of eligibility and compliance certificates before final submission to facilitate a smooth processing experience.
Documents must be submitted in commonly accepted electronic formats such as PDF, DOCX, or XLS. It’s essential to ensure that all documents are clearly labeled and organized to avoid any discrepancies that might hinder the evaluation process.
Payment terms typically involve an advance payment upon signing the contract, followed by milestone payments upon successful deliveries and installation of the machines. Specific details regarding performance security and payment timelines will be outlined in the tender documentation and discussed during the bid clarifications.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders