Department of Health and Family Welfare Tender by Hll Infra Tech Services Limited (GEM/2024/B/5449376)
Cystoscope & Resectoscope set - Pediatric
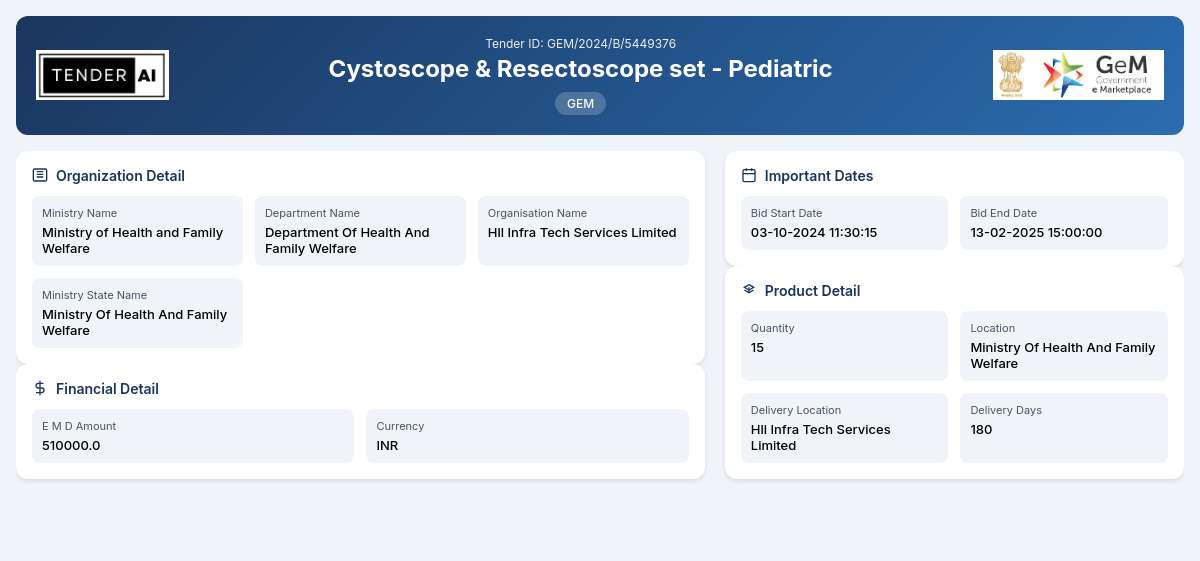
Tender Timeline
Tender Title: Cystoscope & Resectoscope Set - Pediatric
Reference Number: GEM/2024/B/5449376
Issuing Authority/Department: Department of Health and Family Welfare, Ministry of Health and Family Welfare
Scope of Work and Objectives
The objective of this tender is to procure a Cystoscope & Resectoscope set - Pediatric to support healthcare facilities in providing specialized surgical care for pediatric patients. The total quantity required is 15 sets, catering to the demand for advanced medical instrumentation in pediatric surgery.
Eligibility Criteria
Eligible bidders must be registered entities with relevant expertise and experience in manufacturing or supplying medical equipment. The requirements include:
- A minimum of three years of past experience in supplying similar medical devices.
- A minimum average annual turnover of at least 76 Lakh.
- Must comply with solicitation documents and specifications outlined in the tender.
Technical Requirements
Bidders are expected to provide detailed specifications and compliance documentation, including but not limited to:
- OEM (Original Equipment Manufacturer) Authorization Certificate.
- Compliance documents that establish adherence to quality and technical standards.
- Supporting documents validating the specifications of the provided equipment.
For complete technical criteria, bidders may refer to the Technical Specification Document.
Financial Requirements
Each bid must include an Earnest Money Deposit (EMD) amounting to 510,000. This deposit is required to ensure that bidders demonstrate commitment and serious intent towards fulfilling the obligations of the tender.
Document Submission Details
All required documentation must be submitted electronically via the tendering platform as part of the bid. This includes:
- Experience criteria and past performance records.
- Certificates specified in the Additional Terms and Conditions (ATC).
- Compliance with BoQ (Bill of Quantities) specifications.
Special Provisions
- MSE Exemption: Micro, Small, and Medium Enterprises (MSEs) are eligible for exemptions concerning years of experience, as outlined in the tender documents.
- Startup Provisions: Startups may also qualify for exemptions regarding experience and past performance requirements.
Evaluation Process
The bids will undergo a total value-wise evaluation, focusing on the best overall value for the healthcare sector while complying with technical requirements. Key considerations will include:
- Past performance of the bidder.
- Compliance with quality standards.
- Financial viability and pricing competitiveness.
Delivery Locations
Bidders must provide details regarding delivery capabilities and logistics for fulfilling orders to various health facilities across the region, ensuring support for pediatric surgeries nationwide.
Contact Information
For inquiries related to the tender, please refer to the main document for specific contact details. Bidders are encouraged to seek clarifications within the two-day period allowed for technical clarifications during the evaluation phase.
Main Document Link: GeM Bidding Document
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
3 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- Additional Doc 1 (Requested in ATC)
- Additional Doc 2 (Requested in ATC)
- Additional Doc 3 (Requested in ATC)
- Additional Doc 4 (Requested in ATC)
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Corrigendum Updates
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity, having a minimum of three years of past experience in supplying similar medical devices, and achieving a minimum average annual turnover of 76 Lakh.
Bidders must submit an OEM Authorization Certificate, other relevant compliance certificates as specified in the ATC, and documents validating experience and past performance related to similar supplies.
Interested bidders must register on the GeM (Government e-Marketplace) portal, complete the vendor registration process, and ensure that all necessary documentation is in place as specified in the tender document.
Submission is typically required in digital formats such as PDF or Word files, adhering to the specifications set forth in the tender guidelines.
Bidders must adhere to the comprehensive technical specifications outlined in the tender documents, emphasizing quality, efficacy, and compliance with medical instrument standards.
Yes, bidders should ensure their equipment meets recognized international quality standards applicable to medical devices and demonstrate compliance through appropriate certifications.
Bidders must guarantee compliance with all technical prerequisites, including supporting documents that establish adherence to specifications defined in the tender documents.
The EMD required for this tender is 510,000. It is necessary to demonstrate serious intent and ensures commitment by the bidders towards the proposal made.
Upon being awarded the contract, the successful bidder may be required to submit a performance security in a predefined format ensuring fulfillment of contract obligations.
The payment terms are laid out in the tender documents, typically covering advance payments upon order confirmation and milestone payments as deliverables are met.
Price evaluation will focus on comparing total bid values, ensuring that the selected bid offers the best value while meeting all technical requirements.
Bids must be submitted online through the GeM portal following the established procedures, ensuring all required documents are electronically attached.
Bidders should reference the primary document for all deadlines related to bid submission and opening. Awareness of the timeline ensures compliance throughout the bid process.
The evaluation is based on a total value-wise method assessing the bidder’s past performance, compliance with quality standards, and the overall pricing offered.
Notifications regarding the results of the bid evaluation will typically be made via the GeM portal through announcements or individual communications to participating bidders.
MSEs are entitled to exemptions from experience requirements and other supportive measures aimed at enhancing participation in government tenders.
Startups may qualify for benefits similar to MSEs, including exemption from specific requirements regarding experience in the supply of medical devices. This encourages innovative new entrants in the field.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders