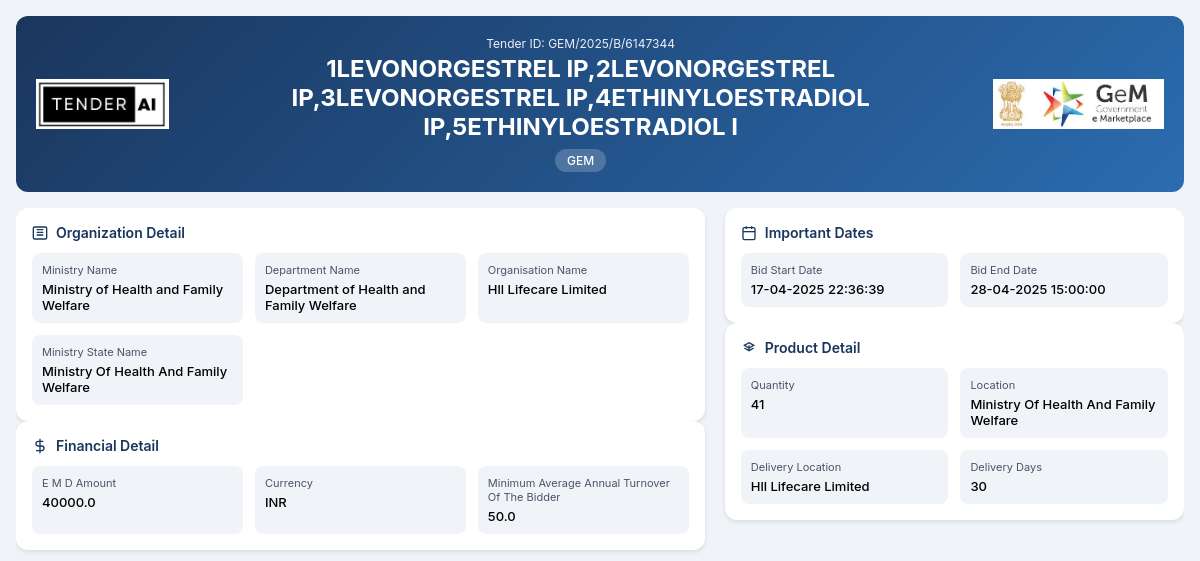
The Department of Health and Family Welfare, under the Ministry of Health and Family Welfare, is inviting proposals for the Supply of API (Active Pharmaceutical Ingredients) as part of its continuous efforts to enhance the availability of essential medicines in the healthcare system. This tender reflects the commitment of the Indian government to ensure that sufficient quantities of pharmaceutical ingredients are readily available for manufacturing a variety of medications that are crucial for public health.
Scope of Work and Objectives
This tender encompasses the procurement of various Active Pharmaceutical Ingredients, particularly LEVONORGESTREL IP and ETHINYLOESTRADIOL IP. The tender aims to establish a reliable supply chain for these essential ingredients, ensuring quality and timely delivery to the designated locations. The overall goal is to support the local pharmaceutical manufacturing sector and enhance the availability of essential drugs in the market.
Eligibility Criteria
Participants must be registered entities with the necessary licenses to engage in the manufacturing and supply of Active Pharmaceutical Ingredients. Interested bidders should demonstrate relevant experience in supplying pharmaceutical inputs, and adherence to Good Manufacturing Practices (GMP) is essential. Compliance with the Department's regulations and certifications of quality standards must be substantiated by appropriate documentation.
Technical Requirements
Bidders must provide documentation confirming that they meet the technical specifications set forth in the tender. Key specifications include:
- Certificates of Analysis
- Ingredient compliance with Indian Pharmacopoeia (IP) standards
- Manufacturing licenses
- Evidence of handling and storage conditions that meet the required quality standards.
Financial Requirements
Bidders must submit a financial proposal that details the pricing for the required quantities. The financial documentation should also highlight any applicable taxes, duties, or additional charges. An Earnest Money Deposit (EMD), which acts as a security deposit for the proposal, is mandatory to ensure bid seriousness.
Document Submission Details
All proposals must be submitted electronically via the designated e-procurement platform. Bidders are responsible for ensuring that all required documents are completed accurately and uploaded prior to the closing date of the tender submission. Late submissions will not be accepted.
Special Provisions
This tender encourages participation from Micro, Small, and Medium Enterprises (MSEs) and provides certain advantages designed to foster inclusivity and support local startups. These provisions include potential relaxation of qualifying criteria, fee waivers, and preference in bidding evaluations for MSEs contributing to 'Make in India' initiatives.
Evaluation Process
Proposals will be evaluated based on predefined criteria including quality, cost-effectiveness, and compliance with technical specifications. A thorough assessment will be conducted to ensure that the submissions align with the governmental requirements and standards.
Delivery Locations
The quantities will need to be delivered to specific locations as outlined in the official documentation accompanying the tender. Attendance to logistics and timely delivery is critical to the success of this procurement initiative.
Contact Information
For further inquiries, bidders may reach out to the official contacts provided in the tender documents. It’s important to ensure all queries are resolved prior to submission deadlines to facilitate compliance and enhance chances of successful bids.
This opportunity presents a critical pathway for suppliers aiming to collaborate with the governmental health sector. Companies meeting the criteria and offering qualified services are strongly encouraged to participate and advance the supply of vital pharmaceutical components.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
5 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC) *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity with the necessary licenses for manufacturing and supplying Active Pharmaceutical Ingredients. Bidders must demonstrate relevant experience in the field of pharmaceuticals, specifically in the production of LEVONORGESTREL IP and ETHINYLOESTRADIOL IP. Adherence to the Good Manufacturing Practices (GMP) and compliance with all regulations set forth by the Department of Health and Family Welfare are imperative. Documentation verifying the above conditions will need to be submitted as part of the proposal.
To qualify for the Supply of API tender, bidders must submit several essential certificates, including:
- Certificates of Analysis for the offered ingredients, ensuring compliance with Indian Pharmacopoeia (IP) standards.
- Valid manufacturing licenses that authorize the production of pharmaceuticals.
- Documented proof of adherence to quality standards that comply with industry regulations. This documentation will be critical in processing tender proposals and ensuring compliance with technical specifications.
The payment terms for the Supply of API will typically involve an initial Earnest Money Deposit (EMD) as a security measure. This deposit assures the authority of the bidder’s seriousness toward fulfilling the procurement contract. Upon successful delivery of the APIs and verification of compliance with specifications, payment will be processed according to the terms established in the finalized contract. Bidders should be prepared to discuss and finalize these terms in their proposal documentation.
Proposals for the Supply of API must be submitted electronically through the designated e-procurement platform. It’s essential that bidders follow all directions as outlined in the tender documentation regarding digital submission, ensuring that all required documents are correctly completed and uploaded prior to the specified closing date. Late or alternative submission methods may result in disqualification.
The tender encourages participation from Micro, Small, and Medium Enterprises (MSEs) by providing several special provisions. These may include relaxed eligibility criteria, fee waivers for participation, and enhanced consideration during the evaluation process. Additionally, MSEs contributing to the ‘Make in India’ initiative may receive preference, fostering local entrepreneurship and sustainability within the pharmaceutical supply chain.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders