Department of Health and Family Welfare Tender by All India Institute Of Medical Sciences (aiims) (GEM/2025/B/5970201)
Fully Automatic ABI/TBI PVR Doppler (14 ports)
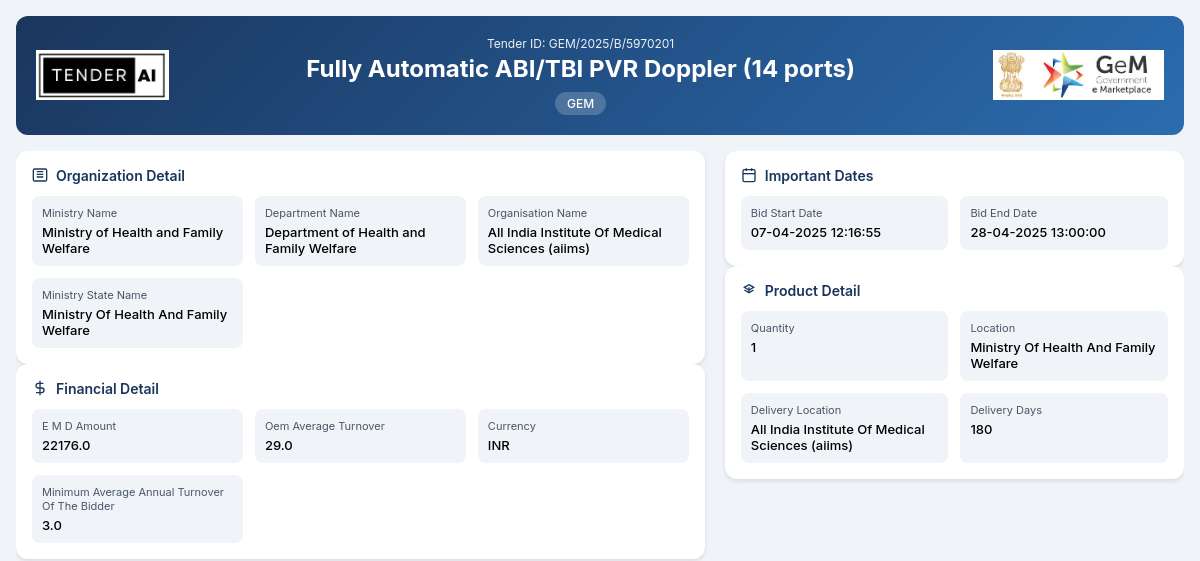
Tender Timeline
Tender Description for Fully Automatic ABI/TBI PVR Doppler (14 ports)
The tender referenced as GEM/2025/B/5970201 has been issued by the Department of Health and Family Welfare, under the Ministry of Health and Family Welfare. This procurement focuses on acquiring a Fully Automatic ABI/TBI PVR Doppler (14 ports) to enhance the capabilities of healthcare facilities.
Scope of Work and Objectives
The main objective of this tender is to procure state-of-the-art medical equipment that allows for accurate assessments of arterial blood flow and can assist in the diagnosis of peripheral vascular diseases. The Fully Automatic ABI/TBI PVR Doppler must be equipped with advanced features, ensuring reliability and efficiency in clinical environments. It should accommodate a total of 14 ports, allowing multiple examinations to be performed simultaneously, thus maximizing patient throughput.
Eligibility Criteria
To participate in this tender, bidders must meet specific eligibility requirements including being a registered entity with valid credentials. Suppliers must demonstrate prior experience in supplying medical devices, especially in the domain of vascular diagnostics. Compliance with health regulations and standards is mandatory.
Technical Requirements
The technical specifications of the ABI/TBI PVR Doppler include high-resolution imaging capabilities, user-friendly interfaces, and compatibility with existing hospital infrastructure. Furthermore, the equipment must adhere to all relevant healthcare quality standards and certifications, ensuring safety and effectiveness in medical procedures.
Financial Requirements
Bidders should prepare to demonstrate their financial viability. Required documents may include balance sheets, profit and loss statements, and any certifications illustrating sound financial management. Financial transparency is crucial and will be a part of the evaluation process.
Document Submission Details
All submissions should be made electronically, adhering to the formats specified in the tender documentation. The completed tender documents must be submitted through the GEM platform for validation. It’s essential that documents are organized and clearly labeled to avoid any discrepancies during review.
Special Provisions
This tender includes special provisions aimed at encouraging participation from Micro, Small, and Medium Enterprises (MSEs) and startups. Vendors classified under these categories will receive additional considerations in the evaluation process, acknowledging their contribution to the economy and innovation.
Evaluation Process
The evaluation will be rigorous, assessing both the technical merits and financial proposals of submitted bids. Criteria for selection will include quality standards, adherence to specifications, delivery timelines, and post-sale support services. The selection committee will also confer weight to proposals that exhibit a commitment to local sourcing in alignment with national policies.
Delivery Locations
Equipment must be delivered to specified health institution locations within the jurisdiction of the Ministry of Health and Family Welfare. The exact delivery points will be confirmed upon contract award.
Contact Information
For any inquiries regarding the tender, vendors are encouraged to reach out through the official communication channels of the Department of Health and Family Welfare. It is imperative to maintain professionalism and clarity in all correspondences related to this procurement process.
In summary, the tender for the Fully Automatic ABI/TBI PVR Doppler (14 ports) is vital for enhancing healthcare delivery within the region. All interested parties are encouraged to review the full tender documentation thoroughly to ensure adherence to requirements and to maximize their chances of successful bid submission.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
3 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Additional Doc 1 (Requested in ATC) *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements for participating in this tender include being a registered entity, demonstrating prior experience in supplying medical equipment, particularly in the vascular diagnostics field, and compliance with necessary health regulations and standards. Bidders must provide documentation validating their registration, experience, and compliance to ensure successful participation in the procurement process.
To meet the technical specifications, bidders must provide relevant certifications such as ISO, FDA approval, or CE marking that validates the quality and safety standards of the Fully Automatic ABI/TBI PVR Doppler. These certificates are critical to ensure that the equipment complies with health guidelines and regulations, thus guaranteeing effective usage in clinical settings.
The performance security may be required post-award, usually amounting to a percentage of the contract value. Bidder submissions should include an Earnest Money Deposit (EMD) to indicate financial commitment. Specific details regarding the amounts and conditions for the EMD will be outlined in the tender documents, ensuring that all participating entities are aware of financial obligations prior to bid submission.
Documents must be submitted electronically via the GEM portal. It is critical that all documents are formatted correctly as per the guidelines provided in the tender. Common acceptable formats may include PDF or other secured document types. Careful attention to submission guidelines will enhance the chance of acceptance during the evaluation process.
Yes, the tender offers specific provisions aimed to promote engagement from Micro, Small, and Medium Enterprises (MSEs) and startups. These benefits include possible relaxations in eligibility criteria, and additional weight in the evaluation process, acknowledging the important role these entities play in the economic landscape and innovation in the health sector. Compliance with ‘Make in India’ policies may also enhance evaluation scores for qualifying bids.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders