Department of Health and Family Welfare Tender by Post Graduate Institute Of Medical Education And Research Chandigarh (GEM/2025/B/6172591)
Nanopore Sequencer with Software and Workstation
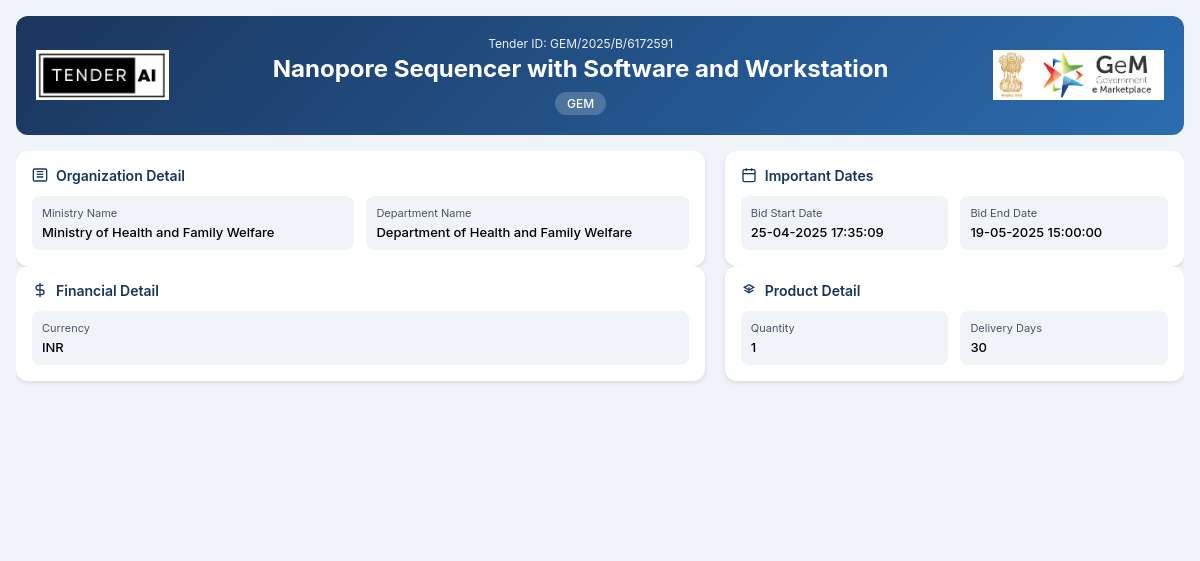
Tender Timeline
Title
Nanopore Sequencer with Software and Workstation
Reference Number
GEM/2025/B/6172591
Issuing Authority
This tender is issued by the Department of Health and Family Welfare, which operates under the Ministry of Health and Family Welfare.
Scope of Work and Objectives
The primary objective of this tender is to procure a Nanopore Sequencer along with its accompanying software and workstation. The Nanopore Sequencer is essential for advanced genomic research and diagnostics, providing high-throughput sequencing capabilities required by health institutions. The successful contractor will be responsible for delivering a complete system that includes both hardware and software components as specified in the tender documentation.
Eligibility Criteria
To qualify for this tender, bidders must possess a valid business registration, demonstrated experience in providing similar equipment, and compliance with any applicable licensing regulations. It is imperative for bidders to demonstrate their capability to meet the technical requirements and warranty provisions outlined in the tender details.
Technical Requirements
Bidders are required to submit detailed specifications of the Nanopore Sequencer, including operational capabilities, configurations, and system compatibility. Compliance with relevant health and safety standards is mandatory, and the equipment must be able to integrate seamlessly within existing laboratory infrastructure.
Financial Requirements
Bidders must provide a detailed price quotation that includes the total cost for the Nanopore Sequencer, software, and workstation, along with any applicable taxes, transportation cost, and warranty provision. The financial proposal should indicate the payment terms and any upfront requirements for performance security.
Document Submission Details
All proposals must be submitted electronically through the designated procurement platform, adhering to any specified document formatting requirements. It is critical for bidders to ensure all required documents, including technical specifications, warranty details, and pricing, are included in the submission.
Special Provisions
There are provisions in place to encourage participation from Micro, Small, and Medium Enterprises (MSEs) and startups. These provisions may include relaxation in eligibility criteria and certain financial benefits.
Evaluation Process
Proposals will undergo a comprehensive evaluation process based on predefined criteria, including technical compliance, cost-effectiveness, and delivery timelines. The final selection will favor proposals that offer the best value for the required specifications and align with the objectives of enhancing health research capabilities.
Delivery Locations
The system must be delivered to specified health institutions across the region as detailed in the tender documents. All logistical arrangements shall be the responsibility of the selected vendor.
Contact Information
While specific contact details are often included in the tender documents, interested bidders should refer to the official procurement website or contact the issuing authority for any clarifications regarding the tender.
By participating in this tender, bidders contribute to enhancing the genomic research capabilities within the healthcare sector, thus impacting public health outcomes positively.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- OEM Authorization Certificate
- Additional Doc 1 (Requested in ATC) *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity, having relevant industry experience, and complying with licensing regulations. Bidders must demonstrate capability in meeting technical specifications and equipment standards required in the tender.
Bidders must submit relevant certificates including business registration, ISO certifications (if applicable), and compliance documentation related to health and safety standards for the Nanopore Sequencer.
The registration process involves submitting all required documentation through the specified procurement platform. Bidders must ensure that the submissions meet the technical and financial requirements detailed in the tender announcement.
The payment terms generally include an upfront payment followed by milestone payments based on the successful delivery and installation of the Nanopore Sequencer. Details will vary and should be specified in the financial proposal.
Yes, the tender includes special provisions for MSEs, which may involve simplified eligibility criteria, financial incentives, and potential advancements when competing against larger entities. These provisions aim to support local businesses and enhance participation in public procurement.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders