Department of Health and Family Welfare Tender by All India Institute Of Medical Sciences (aiims) (GEM/2025/B/6176708)
Intermittent Pneumatic Compression Device (V2)
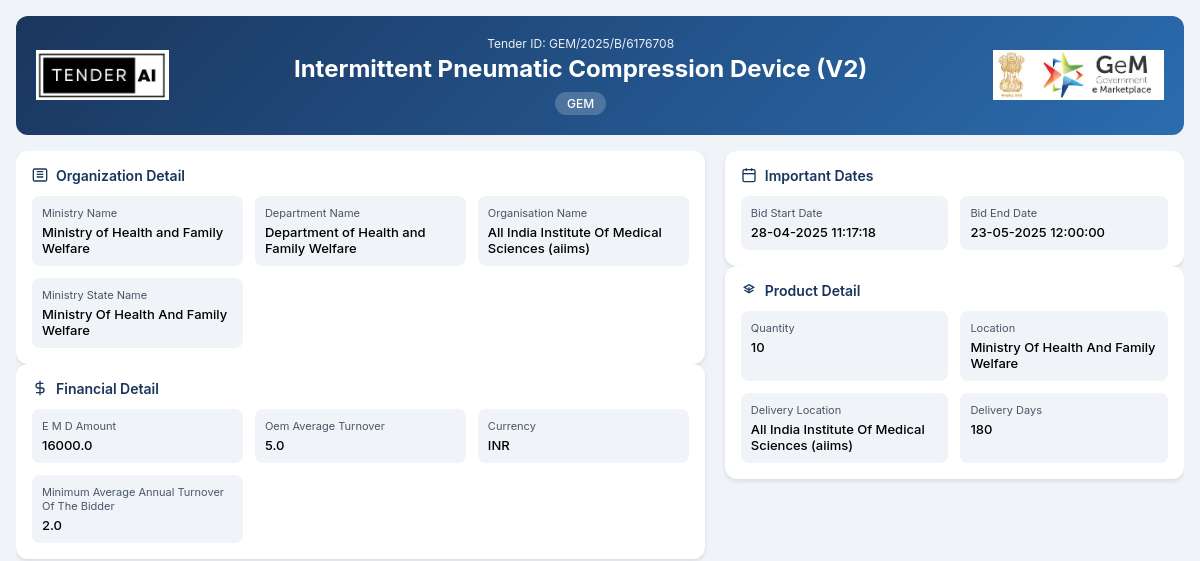
Tender Timeline
Tender Title: Intermittent Pneumatic Compression Device (V2)
Reference Number: GEM/2025/B/6176708
Issuing Authority/Department:
The tender is utilized by the Department of Health and Family Welfare, under the supervision of the Ministry of Health and Family Welfare. This esteemed department focuses on improving healthcare services and products, ensuring the provision of quality medical equipment.
Scope of Work and Objectives
This tender aims to procure a total of 10 units of the Intermittent Pneumatic Compression Device (V2). These devices are essential to enhance patient care, particularly for those in need of improved blood circulation and reduced risk of deep vein thrombosis. The objectives encompass:
- Ensuring high-quality medical devices that adhere to strict health standards.
- Facilitating smoother patient recovery and enhancing overall treatment outcomes.
- Supporting healthcare facilities with reliable equipment that meets operational demands.
Eligibility Criteria
To participate in the tender, bidders must meet the following eligibility criteria:
- Must be a fully registered entity authorized to operate in the medical equipment sector.
- Proven experience in supplying similar medical equipment.
- Compliance with relevant health and safety regulations.
- Ability to provide necessary certifications that validate the quality and safety of the devices.
Technical Requirements
Participants must ensure the Intermittent Pneumatic Compression Device (V2) meets the following technical requirements:
- The devices should be designed to provide effective intermittent compression for therapeutic purposes.
- Must have adjustable settings to cater to different patient needs concerning compression intensity and duration.
- Should possess certification demonstrating compliance with health and safety standards.
Financial Requirements
Bidders are required to present complete financial documentation to substantiate their financial capacity to execute the contract:
- Detailed cost breakdown for the devices including all associated costs (e.g., delivery, installation).
- Financial statements from the last three years demonstrating solvency and reliability.
Document Submission Details
Bid submissions must include the required documents organized systematically. All documents should be submitted electronically through the designated tender portal. They must include:
- Completed tender forms.
- Technical specifications of the medical devices.
- Financial proposals.
- Any relevant certificates that validate compliance.
Special Provisions
This tender may include provisions to support Micro, Small, and Medium Enterprises (MSEs). Eligible small companies may receive beneficial conditions regarding registration and bidding processes, encouraging competitive participation from diverse suppliers.
Evaluation Process
The evaluation of bids will focus on a comprehensive and transparent approach:
- Technical evaluation will assess compliance with specifications and requirements.
- Financial evaluation will consider pricing efficiency, ensuring best value for the government.
- Weightage may be provided to bids submitted by MSEs and startups as per applicable policies.
Delivery Locations
The devices are to be delivered to designated healthcare centers as stipulated by the Department of Health and Family Welfare. Bidders should include logistical plans for prompt and efficient delivery in their proposals.
Contact Information
For additional queries regarding the tender specifications or submission processes, please refer to the official website of the Department of Health and Family Welfare for their contact resources.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Additional Doc 1 (Requested in ATC) *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity capable of supplying medical devices, having previous experience in the healthcare equipment sector, and compliance with healthcare regulatory standards. Bidders must also submit certifications proving the quality and safety of the Intermittent Pneumatic Compression Device (V2).
Bidders must possess valid certifications that validate compliance with health and safety regulations. This may include ISO certifications, CE marking, or any other relevant documentation proving that the Intermittent Pneumatic Compression Device (V2) meets required standards for quality and safety.
Payment terms will be articulated in the bid documents, typically requiring performance security, an Earnest Money Deposit (EMD), and specifications regarding milestones for payment releases. Ensuring clarity on these aspects is crucial for bidders during the financial proposal stage.
Bidders must submit their proposals electronically through the designated tender portal. This submission must include all required documents, including technical specifications and financial proposals for the Intermittent Pneumatic Compression Device (V2), ensuring compliance with submission guidelines.
Yes, there are special provisions for Micro, Small, and Medium Enterprises (MSEs) to promote their participation in this tender. The tender may grant benefits such as relaxed eligibility criteria, offering enhanced opportunities for small businesses to contribute to the procurement process while adhering to government policies.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders