Department of Health Research Tender by Indian Council Of Medical Research (icmr) (19d9490a)
Calibration Services - Medical; Mass and Volume, Pressure and Vacuum, Temperature, Specific heat &
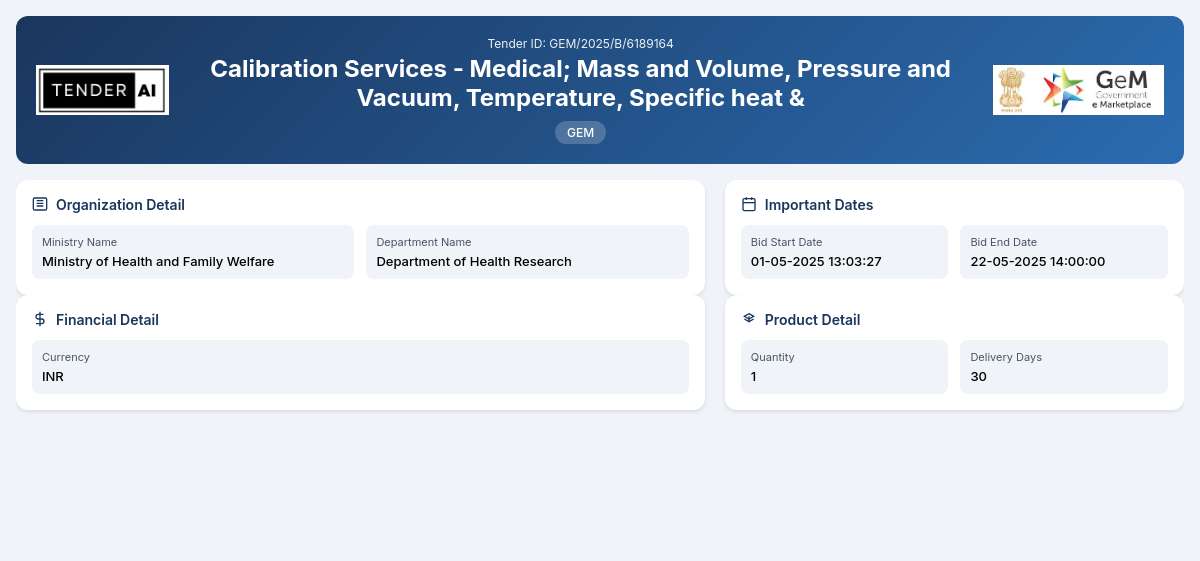
Tender Timeline
Tender Title: Calibration Services - Medical
Reference Number: 19d9490a
Issuing Authority: Department of Health Research, Ministry of Health and Family Welfare
Scope of Work and Objectives
The primary objective of this tender is to procure calibration services for medical instruments. This includes but is not limited to calibration of mass and volume, pressure and vacuum, temperature, and other related parameters within specified environments. The service is intended to maintain compliance with health standards and improve operational efficiencies within healthcare settings. Bidders will be expected to demonstrate their capability in providing these precision calibration services effectively.
Eligibility Criteria
To participate in this tender, bidders must meet specific eligibility requirements. Qualified entities must be registered participants in the appropriate medical calibration sectors with a focus on compliance with relevant standards. The demonstration of prior successful calibration projects in the medical domain will be essential.
Technical Requirements
Proposals should outline adherence to technical specifications that mandate calibration accuracy according to industry standards. Bidders are required to demonstrate their technical capabilities, detailing their experience with the calibration of medical instruments, as well as any certifications from recognized authorities such as NABL (National Accreditation Board for Testing and Calibration Laboratories).
Financial Requirements
Bidders must provide a clear breakdown of pricing as part of their submissions. This breakdown should include all necessary costs, emphasizing transparency in the financial requirements associated with providing the calibration services.
Document Submission Details
Submission of required documents is critical in maintaining compliance with the tendering process. Bidders are advised to submit all relevant documentation electronically or physically as specified in the guidelines. The necessary documents include company registration, certifications, and detailed proposals outlining service capabilities.
Special Provisions
The tender does consider provisions for Micro, Small and Medium Enterprises (MSEs), which may include financial assistance or incentives conducive to fostering participation from smaller business entities. Startups are also encouraged to participate under special provisions aimed at promoting innovation and new entrants into the calibration sector.
Evaluation Process
The evaluation of submissions will follow criteria that prioritize technical competence, financial viability, and compliance with established standards. EMD (Earnest Money Deposit) details must be adhered to for submission acceptance. Evaluation will be conducted in a structured manner, ensuring transparency and equity throughout.
Delivery Locations
Services must be delivered onsite at designated healthcare facilities across the specified region, adhering to any logistical requirements laid out in the tender documents.
Contact Information
For further inquiries regarding this tender, bidders can reach out to the respective department's contact points, ensuring all questions are directed appropriately to facilitate clarity in the submission process.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Certificate (Requested in ATC)
- Additional Doc 1 (Requested in ATC) *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity capable of providing medical calibration services. Additionally, bidders should demonstrate prior experience in this field and compliance with health standards.
Bidders are required to present valid certifications that comply with industry regulations, such as NABL accreditation, to validate their technical qualifications and adherence to quality standards in the calibration of medical instruments.
Proposals must be submitted in the prescribed format as indicated in the tender documents. This could involve electronic submissions or physical deliveries, ensuring all required documents, including financial proposals and service capabilities, are included.
Bidders must note the Earnest Money Deposit (EMD) requirements as well as conditions surrounding performance security. Clear understanding around payment terms is essential, ensuring budgetary compliance throughout the project’s lifecycle.
Yes, there are provisions favorable for MSEs which may include relaxed eligibility criteria, financial assistance, or priority evaluation processes to encourage their participation, aligning with broader initiatives like ‘Make in India’.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders