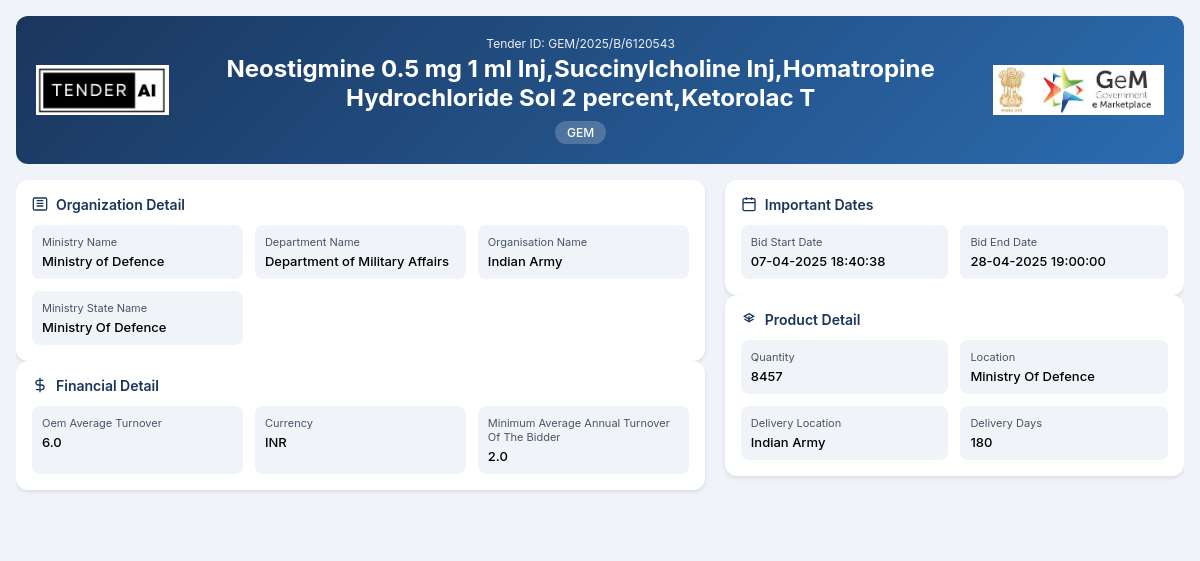
Tender Title: Neostigmine Inj
Reference Number: GEM/2025/B/6120543
Issuing Authority: Department of Military Affairs, Ministry of Defence
The Department of Military Affairs under the Ministry of Defence is inviting bids for the procurement of various pharmaceutical products, including Neostigmine 0.5 mg 1 ml Inj, Succinylcholine Inj, and Homatropine Hydrochloride Sol, among others. This tender aims to ensure the availability of essential medications for military and healthcare applications, facilitating adequate healthcare support for defense personnel.
Scope of Work and Objectives
The primary goal of this tender is to procure a total quantity of 8,457 units of specified pharmaceutical products. This includes:
- Neostigmine 0.5 mg 1 ml Inj
- Succinylcholine Inj
- Homatropine Hydrochloride Sol 2 percent
- Ketorolac T
- Lignocaine Hydrochloride 4 percent topical solution (30 ml bottles)
The scope of work encompasses sourcing, quality assurance, delivery, and any associated logistics to ensure timely availability of these products to designated military units and healthcare facilities.
Eligibility Criteria
To be considered for this tender, bidders must meet the following eligibility requirements:
- Registered as a legal entity in India
- Possess the requisite licensing for the sale of pharmaceutical products
- Demonstrated experience in supplying similar products to government or military clients
Technical Requirements
Bidders must ensure that all products conform to the latest pharmacological standards and quality-controlled tests. Furthermore, the technical requirements involve:
- Adherence to specified product dosages and formulations
- Compliance with recognized national quality standards for pharmaceuticals
- Documentation of sourcing and handling processes
Financial Requirements
Potential vendors are required to exhibit financial stability through:
- Submission of recent financial statements
- Evidence of the ability to fulfill the contract amount without financial strain
Document Submission Details
All documentation must be submitted electronically through the specified government procurement portal before the official deadline. Final submissions should include:
- Technical bid documents
- Financial bid documents
- Compliance certificates
Special Provisions
This tender includes provisions for Micro, Small, and Medium Enterprises (MSEs) and provides benefits to startups. Eligible startups or MSEs can avail themselves of certain incentives that encourage participation in these tenders, promoting local economies and fostering innovation.
Evaluation Process
The evaluation of bids will be conducted based on criteria that include:
- Technical compliance
- Financial competitiveness
- Quality assurance provisions
Bids will be evaluated in a structured process to ensure prompt and fair adjudication.
Delivery Locations
Successful bidders will be required to deliver defined quantities of the pharmaceutical products to various military bases and healthcare facilities across designated geographical locations specified in the tender documents.
Contact Information
For any inquiries regarding the tender, interested parties should contact the Department of Military Affairs for clarifications and further information. Specific contact details can be accessed through the official government procurement portal.
The successful fulfillment of this tender means a significant improvement in the medical resources available to the defense sector, ensuring the health and readiness of personnel.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity with necessary licenses for pharmaceutical distribution, proven experience in similar supply contracts, and compliance with national healthcare regulations. Bidders must submit valid proof of their eligibility during the application process.
All products must comply with established national quality standards applicable to pharmaceuticals, including formulation, dosage, and packaging requirements. Adequate documentation certifying compliance with these technical specifications is mandatory.
The EMD is a financial assurance that potential bidders must provide to secure their participation. It is typically expressed as a percentage of the total bid value and is aimed at ensuring bidders’ commitment to the procurement process.
Bids must be submitted electronically via the official government procurement portal. All required documents, including both technical and financial bids, must be uploaded in the specified formats, adhering to the tender submission guidelines.
Yes, the tender includes provisions designed to support MSEs and promote their participation through financial incentives, reduced documentation requirements, and a preference in the evaluation and selection process.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders