Department of Military Affairs Tender by Indian Army (GEM/2025/B/6156063)
Albumin test Kit 10X44 ML for ERBA XL 200,AHG,Alkaline Phosphate test kit 2X44 ML for ERBA XL 200,A
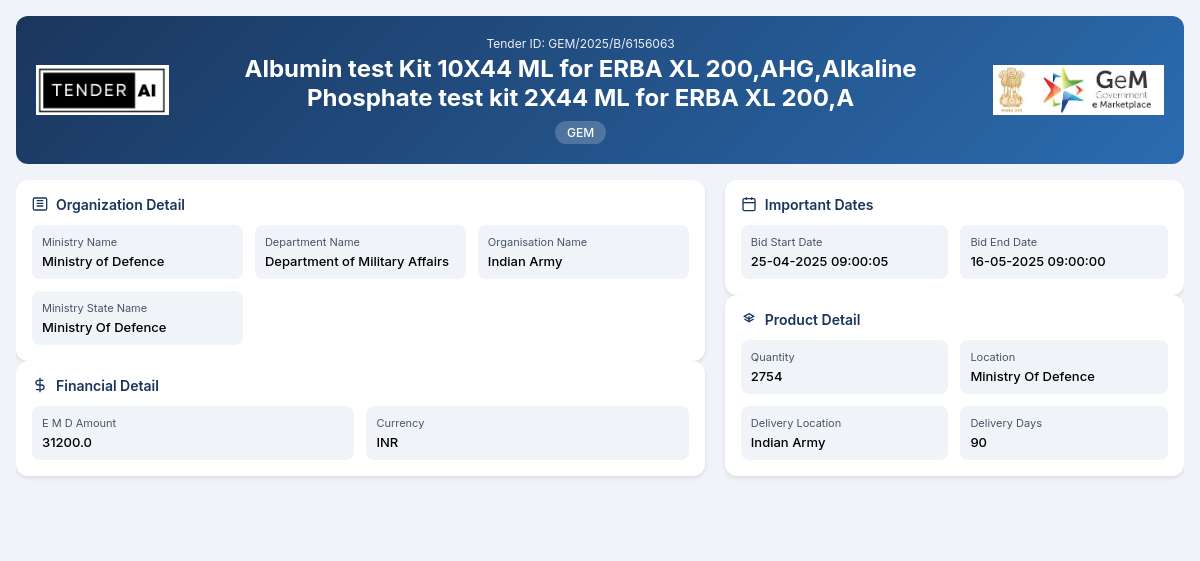
Tender Timeline
Tender Title: LAB REAGENTS
Reference Number: GEM/2025/B/6156063
Issuing Authority/Department
Department of Military Affairs, Ministry of Defence
Scope of Work and Objectives
The primary objective of this tender is to procure LAB REAGENTS, which include various diagnostic kits essential for laboratory testing. The required items consist of:
- Albumin test Kit 10X44 ML for ERBA XL 200
- Alkaline Phosphate test kit 2X44 ML for ERBA XL 200
- Additional tests such as Amalyse test kit, Anti Sera kits, Bilirubin Direct and Total test kits, and various others for comprehensive laboratory diagnostics.
The total quantity for this procurement is 2754 units, aiming to enhance laboratory capabilities and testing accuracy across medical fields.
Eligibility Criteria
To be eligible for this tender, vendors must:
- Be a registered entity with pertinent government registrations.
- Maintain compliance with the quality and technical specifications outlined in the tender documentation.
- Demonstrate prior experience in handling medical laboratory supplies.
- Provide relevant certifications and documentation showcasing capability and reliability in delivering medical reagents.
Technical Requirements
Vendors must fulfill the following technical requirements:
- All LAB REAGENTS should conform to the prescribed quality standards and specifications tailored for the ERBA XL 200 laboratory equipment.
- Must include comprehensive documentation regarding the functionality and validation of the provided reagents.
- All items must include validity and quality assurance certificates.
Financial Requirements
Participants should detail their financial stability and ability to deliver on the project. The financial proposals must consist of:
- A breakdown of costs for each reagent.
- Clear stipulations regarding payment terms based on delivery milestones.
Document Submission Details
Prospective bidders are required to submit their proposals along with all necessary documentation:
- Financial bids in a secure format.
- Technical specifications and compliance documents.
- Submission should strictly adhere to the formatting requirements as described in the tender guidelines.
Special Provisions
This tender encourages participation from Micro, Small, and Medium Enterprises (MSEs) and offers various benefits, enhancing opportunities for startups in the healthcare sector. Vendors falling under these categories will be given preference during the evaluation process.
Evaluation Process
The evaluations will be conducted based on:
- Compliance with technical specifications.
- Financial viability of bids.
- Historical performance in similar procurements.
- Any additional certifications that demonstrate product reliability and compliance with health regulations.
Delivery Locations
The delivery for the goods will be specified in the contract and depend on the requirements outlined by the Ministry of Defence. All deliveries must adhere to logistical and safety standards that pertain to medical equipment.
Contact Information
For further clarifications regarding this tender, you may contact the Department of Military Affairs, Ministry of Defence, directly. It is advisable to communicate through formal channels provided in the tender documents.
By participating in this tender, vendors contribute significantly to improving public health through enhanced laboratory testing capabilities. Each applicant is encouraged to meticulously review the documents and ensure compliance with all outlined requirements to facilitate a successful tendering process.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Compliance of BoQ specification and supporting document *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Similar Tenders
Frequently Asked Questions
The eligibility requirements for this tender include being a registered entity with necessary government certifications. Vendors must exhibit prior experience in supplying medical laboratory supplies and demonstrate compliance with all technical specifications outlined.
Vendors are required to provide a range of certificates including:
- Product quality assurance certificates.
- Compliance certificates regarding operational standards for medical reagents.
- Any relevant ISO certifications that demonstrate adherence to medical supply regulations.
The registration process typically requires bidders to ensure they have the appropriate government registrations. They should then follow the guidelines provided in the tender documentation to submit their bids correctly.
All documents must be submitted in electronic formats, specifically PDF or ZIP formats, to ensure compatibility. Bidders should adhere to any specific format requirements set out in the tender guidelines.
The payment terms will be established based on milestones related to the delivery of the reagents. Bidders need to clearly articulate their financial proposal, including terms on advance payment, partial payments upon delivery, and final settlement.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders