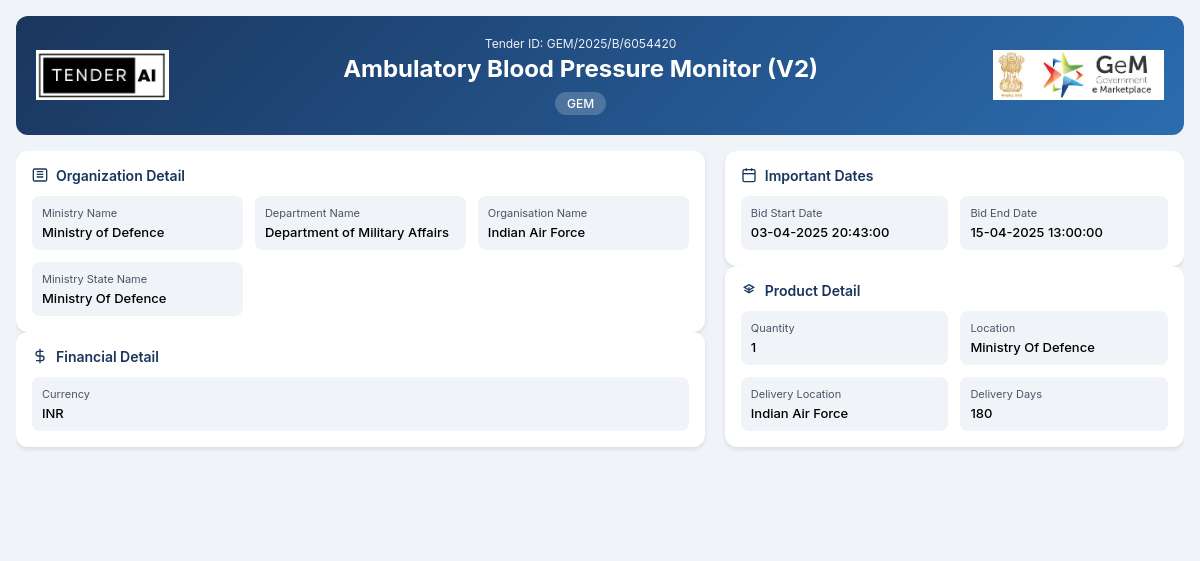
Tender Title: Ambulatory Blood Pressure Monitor (V2)
Tender Reference Number: GEM/2025/B/6054420
Issuing Authority: Department of Military Affairs, Ministry of Defence
The Ambulatory Blood Pressure Monitor (V2) tender seeks qualified vendors to supply the specified medical equipment to enhance healthcare services. This tender represents a strategic move in equipping healthcare facilities with advanced technology to monitor patients' blood pressure continuously, thereby improving diagnostic accuracy and patient care.
Scope of Work and Objectives
The primary objective of this tender is to procure an Ambulatory Blood Pressure Monitor (V2) that meets rigorous clinical standards. The awarded vendor will be responsible for supplying, delivering, and providing necessary support for the equipment. The scope includes:
- Delivery of [X] units of Ambulatory Blood Pressure Monitors.
- Installation and operational training for healthcare staff.
- Providing after-sales support and maintenance.
Eligibility Criteria
The eligibility criteria for this tender include:
- Vendors must be registered entities with a valid license to operate within the medical equipment sector.
- Proven track record of supplying similar medical devices in the past.
- Compliance with prevailing health and safety regulations.
Technical Requirements
Vendors must ensure that the products meet the following technical specifications:
- Monitors with accurate and reliable blood pressure readings.
- User-friendly interface and connectivity options.
- Compliance with national and international quality standards.
Financial Requirements
Bidders are required to submit a detailed financial proposal, including:
- Pricing for each unit of the Ambulatory Blood Pressure Monitor.
- Any additional costs, such as installation or training.
- Payment terms and conditions must be clearly outlined.
Document Submission Details
All necessary documents and proposals should be submitted electronically via the designated tender portal before the submission deadline. Required documents include:
- Company registration certificate.
- Technical specifications compliance sheet.
- Financial bid document.
Special Provisions
The tender may include special provisions for Micro, Small, and Medium Enterprises (MSEs) and startups. Applicants qualifying as MSEs are encouraged to identify themselves in their submissions to benefit from preferential evaluation.
Evaluation Process
The evaluation of submitted tenders will be conducted based on:
- Compliance with technical specifications and requirements.
- Financial viability and pricing structure.
- Overall capability and experience of the supplier.
Delivery Locations
The final delivery points for the Ambulatory Blood Pressure Monitors will be specified upon the award of the tender and will typically include hospitals and healthcare facilities serviced by the Department of Military Affairs.
Contact Information
For further inquiries related to this tender, vendors are encouraged to reach out to the Department of Military Affairs. Specific contact details, if available, can be obtained from the official tender portal.
In conclusion, this tender not only aims to procure high-quality medical equipment but also underscores the commitment of the Ministry of Defence to enhance healthcare service delivery through technological advancements. Vendors are urged to meet all outlined requirements meticulously for successful submission and consideration.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Certificate (Requested in ATC)
- OEM Authorization Certificate *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Corrigendum Updates
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity with a valid operating license in the medical equipment sector, demonstrating experience in supplying similar devices, and compliance with all relevant health and safety regulations. Vendors must also provide documents confirming their work history and technical capability in delivering similar products.
All bidders must submit a company registration certificate, valid licenses for operating within the medical industry, and certifications evidencing compliance with applicable quality standards. Any relevant technical certifications for the Ambulatory Blood Pressure Monitor must also be included to ensure alignment with industry regulations.
The registration process typically involves creating an account on the designated tender portal. Bidders must submit their company’s details and upload required documentation as specified in the tender requirements. Once registered, vendors can submit their proposals electronically before the cutoff time.
Bidders are usually required to submit documents in PDF or Word format for technical and financial proposals. All documents must be clear and legible, ensuring compliance with the submission guidelines outlined in the tender documentation.
The tender includes special provisions to support Micro, Small, and Medium Enterprises (MSEs) and startups. MSEs may receive preferential evaluation, helping them compete effectively for this Ambulatory Blood Pressure Monitor (V2) tender. Additionally, startups can leverage the ‘Make in India’ policies to favor their participation, promoting local production and procurement.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders