Tender Timeline
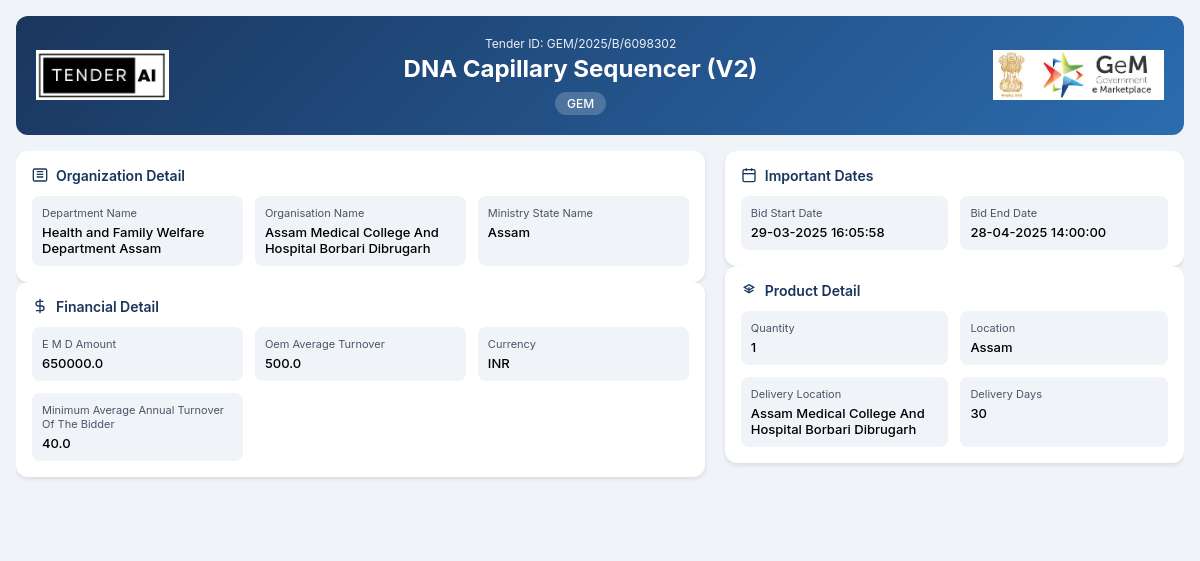
Tender Title: DNA Capillary Sequencer (V2)
Reference Number: GEM/2025/B/6098302
Issuing Authority/Department: Health and Family Welfare Department Assam
The DNA Capillary Sequencer (V2) tender is an initiative by the Health and Family Welfare Department Assam to procure advanced DNA sequencing technology aimed at enhancing various medical research capabilities and diagnostic services. The fundamental objective of this tender is to ensure the acquisition of a state-of-the-art DNA capillary sequencer that offers high throughput and accuracy in genetic analysis.
Scope of Work and Objectives
The scope of work under this tender includes the supply, installation, and commissioning of the DNA Capillary Sequencer (V2). The supplier is expected to deliver a machine that meets specified technical requirements, provides user training, and ensures post-sales support for a specified duration. The objectives include improving testing efficiency and accuracy for genetic studies, thus bolstering the state's healthcare capabilities.
Eligibility Criteria
To qualify for this tender, bidders must meet certain eligibility requirements including but not limited to being a registered and operational entity in India with adequate experience in supplying laboratory instruments. Vendors must also demonstrate financial stability and have previous experience in similar installations.
Technical Requirements
The DNA sequencer must fulfill stringent technical specifications such as high throughput capabilities, compatibility with existing laboratory setups, advanced software for data analysis, and compliance with industry standards. Bidders are required to provide comprehensive documentation regarding the technical attributes of their offered devices.
Financial Requirements
Bidders must provide a clear financial proposal aligned with the specifications. They are expected to include quotes that detail costs for equipment, installation, and any associated services. Detailed breakdowns of proposed warranties and post-purchase support terms should also be incorporated.
Document Submission Details
Interested parties should submit their proposals electronically through the designated government e-marketplace portal. All submissions must include required documents such as technical specifications, financial quotes, company registration details, and any necessary statutory certifications.
Special Provisions
This tender encourages participation from Micro, Small, and Medium Enterprises (MSEs) and startups. Specific incentives such as price preference may be extended to promote their involvement, facilitating fair competition and supporting small business growth in the healthcare sector.
Evaluation Process
The evaluation will follow a structured process that assesses both technical and financial aspects of bids. The technical proposals will be evaluated for compliance with specifications, whereas the financial proposals will be analyzed for cost-effectiveness and transparency. A two-stage evaluation approach will be adopted where only technically qualified bidders will proceed to the financial bid stage.
Delivery Locations
The delivery of the DNA Capillary Sequencer is expected to be made at predetermined locations specified by the Health and Family Welfare Department in Assam. Bidders should ensure prompt delivery and adherence to project timelines.
Contact Information
For more information regarding the DNA Capillary Sequencer (V2) tender, bidders may refer to the official portal or reach out to the Health and Family Welfare Department Assam’s procurement office for any inquiries or clarifications.
General Information
Financial Information
Evaluation and Technical Information
Tender Documents
4 DocumentsDocuments Required from Seller
- Experience Criteria
- Past Performance
- Bidder Turnover
- Certificate (Requested in ATC)
- OEM Authorization Certificate
- OEM Annual Turnover
- Additional Doc 1 (Requested in ATC) *In case any bidder is seeking exemption from Experience / Turnover Criteria
- the supporting documents to prove his eligibility for exemption must be uploaded for evaluation by the buyer
Corrigendum Updates
Similar Tenders
Frequently Asked Questions
The eligibility requirements include being a registered entity with valid documentation, demonstrating relevant experience in supplying similar laboratory instruments, and maintaining compliance with applicable legal standards. Bidders must showcase financial stability as evidenced by their balance sheets and tax compliance.
The technical specifications for the DNA Capillary Sequencer include high throughput processing capabilities, precise genetic analysis features, integration compatibility with existing laboratory systems, and robust software solutions for data interpretation. A detailed proposal must outline these specifications thoroughly.
The payment terms typically involve an initial deposit upon contract signing, followed by staggered payments contingent upon the successful installation and commissioning of the equipment. Specific terms will be outlined in the tender documents, ensuring clarity on payment schedules and linked deliverables.
Submission methods include electronic submissions through the official government e-marketplace platform, where all necessary documents must be uploaded in specified formats. Timely submission is crucial to ensure consideration under the established deadlines set forth in the tender documentation.
Micro, Small, and Medium Enterprises (MSEs) are often granted provisions like price preference and additional support during the assessment process. This tender encourages MSE participation by ensuring equal opportunities and robust competition, which aligns with national goals of promoting local enterprises in line with ‘Make in India’ policies.
Probable Bidders
Get Tender Alerts
Get notifications for similar tenders